Thiazolobenzyne: a versatile intermediate for multisubstituted benzothiazoles†
Abstract
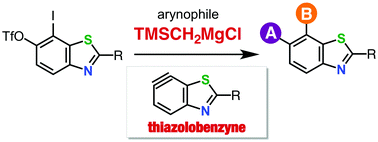
Thiazolobenzyne, which is a benzyne species fused with a thiazole ring, was efficiently generated via an iodine–magnesium exchange reaction of an ortho-iodoaryl triflate-type precursor using a trimethylsilylmethyl Grignard reagent as an activator. A wide range of arynophiles reacted efficiently with the thiazolobenzynes generated by this method to afford various multisubstituted benzothiazoles.

 Please wait while we load your content...
Please wait while we load your content...