Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The organocatalytic ring-opening polymerization of N-tosyl aziridines by an N-heterocyclic carbene†
Camille
Bakkali-Hassani
a,
Elisabeth
Rieger
b,
Joan
Vignolle
a,
Frederik R.
Wurm
 *b,
Stéphane
Carlotti
*a and
Daniel
Taton
*a
*b,
Stéphane
Carlotti
*a and
Daniel
Taton
*a
aLaboratoire de Chimie des Polymères Organiques (LCPO), Université de Bordeaux, IPB-ENSCBP, 16 av. Pey Berland, 33607 PESSAC cedex, France. E-mail: carlotti@enscbp.fr; taton@enscbp.fr; Fax: +33 (0)5 40 00 84 87; Tel: +33 (0)5 40 00 84 86
bMax-Planck-Institut für Polymerforschung (MPI-P), Ackermannweg 10, D-55128 Mainz, Germany. E-mail: wurm@mpip-mainz.mpg.de
First published on 6th July 2016
Abstract
The ring-opening polymerization of N-tosyl aziridines, in the presence of 1,3-bis(isopropyl)-4,5(dimethyl)imidazol-2-ylidene as an organocatalyst and an N-tosyl secondary amine as initiator mimicking the growing chain, provides the first metal-free route to well defined poly(aziridine)s (PAz) and related PAz-based block copolymers.
Owing to their near unlimited structural diversity allowing their steric and electronic properties to be finely tuned, N-heterocyclic carbenes (NHCs) have revolutionized organometallic chemistry as ligands for transition metals in the last two decades.1 NHCs have also gained increasing popularity as true organic catalysts in molecular chemistry.1,2 Polymer synthesis has also greatly benefited from the potential of NHCs, providing a straightforward and metal-free synthetic strategy to a wide range of polymers.3 Cyclic esters (e.g.D,L-lactide and lactones) have been by far the most investigated monomers for the NHC-organocatalyzed ring-opening polymerization (OROP).3,4 The range of monomers amenable to polymerization by NHC catalysis has also been expanded, not only to the ROP of other heterocycles5 (e.g. oxiranes, cyclic carbonates, carbosiloxanes), but also to the group transfer polymerization of alkyl (meth)acrylates,6 and to some step-growth polymerizations.7 Besides their role as catalysts, a few NHCs can also serve as direct nucleophilic initiators, for chain-growth polymerization reactions, either through ring-opening of heterocyclic monomers,8 such as lactide, N-carboxyanhydrides or lactams, or through 1,4-conjugate addition of some (meth)acrylics.9
In this communication, we demonstrate the first OROP of N-activated aziridines using an NHC, namely, 1,3-bis(isopropyl)-4,5(dimethyl) imidazol-2-ylidene (Me5-Ipr). While aziridines and oxiranes are isoelectronic, the two types of monomers behave very differently in ROP. For instance, the simplest representative of each family, i.e. ethylene oxide and aziridine (or azacyclopropane) is polymerized by an anionic mechanism and a cationic mechanism, respectively. While poly(ethylene oxide) can be prepared by anionic means in a controlled fashion, the cationic ROP of aziridine is accompanied by chain transfer reactions to the polymer, forming hyperbranched poly(ethylene imine) (PEI) with a broad dispersity (Đ). Interestingly, Toste, Bergman et al. have reported in 2005 that 2-n-alkyl-N-sulfonylaziridines can be subjected to a controlled anionic ROP through monomer activation by N-tosylation.10 Typically, the ROP of the N-sulfonylaziridine is performed at 50 °C in DMF, in the presence of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of N-alkyl-methanesulfonamide
1 molar ratio of N-alkyl-methanesulfonamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KHMDS as an initiating system, which affords substituted polyaziridines (PAz) of narrow molar mass distribution. The corresponding linear 2-n-alkyl substituted PEI derivatives can next be obtained upon deprotection of the N-sulfonyl moieties, under mild conditions.10,11 Ensuing reports by Wurm et al. have resorted to the living anionic ROP pathway to achieve “in-chain” functional PAz incorporating vinyl or acetal moieties, from purposely designed functional N-sulfonyl aziridines.12
KHMDS as an initiating system, which affords substituted polyaziridines (PAz) of narrow molar mass distribution. The corresponding linear 2-n-alkyl substituted PEI derivatives can next be obtained upon deprotection of the N-sulfonyl moieties, under mild conditions.10,11 Ensuing reports by Wurm et al. have resorted to the living anionic ROP pathway to achieve “in-chain” functional PAz incorporating vinyl or acetal moieties, from purposely designed functional N-sulfonyl aziridines.12
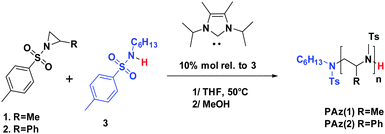
As mentioned, this N-sulfonylaziridine ROP method employs a stoichiometric amount of KHMDS rel. to the N-activated amine. Consequently, it also introduces metallic residues in the final PAz compounds. Here, the Me5-Ipr NHC is used in a true catalytic amount (10% mol rel to the amine initiator). We thus provide a facile OROP synthesis to well-defined metal-free PAz via a novel NHC catalytic pathway, as depicted in Scheme 1. We also report the first examples of all PAz-based block copolymers by sequential Me5-Ipr-mediated OROP of two different N-sulfonylaziridines.
Both 2-methyl-N-p-toluenesulfonyl aziridine (1) and 2-phenyl-N-p-toluenesulfonyl aziridine (2) were investigated as monomer substrates for the Me5-Ipr-OROP. The polymerization of (1) was first carried out at 50 °C in THF -instead of DMF used in previous work10,12- in the presence of N-hexyl-p-toluenesulfonylamine (3) as an initiator and Me5-Ipr as a catalyst (Scheme 1). Under these conditions, well-defined PAz(1) with excellent control over molar masses (up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and low dispersities (Đ < 1.10) were obtained (entries 1–4, Table 1 and Fig. S1, ESI†). While 100 eq. of 1 could be quantitatively converted within 24 h, 5 days were needed to reach completion of the OROP of 2, due to the effect of steric hindrance of the 2-substituent on the aziridine ring on monomer reactivity. Yet, PAz(2) exhibiting molar masses increasing with the initial [2]0/[3]0 molar ratio (entries 5–7, Table 1) were also achieved in this case. In contrast to PAz(1), however, a small shoulder progressively appeared in the high molar mass region of SEC traces of PAz(2) (Fig. S2 in the ESI†), as the initial [2]0/[3]0 was increased, i.e. for higher molar masses targeted.
000 g mol−1) and low dispersities (Đ < 1.10) were obtained (entries 1–4, Table 1 and Fig. S1, ESI†). While 100 eq. of 1 could be quantitatively converted within 24 h, 5 days were needed to reach completion of the OROP of 2, due to the effect of steric hindrance of the 2-substituent on the aziridine ring on monomer reactivity. Yet, PAz(2) exhibiting molar masses increasing with the initial [2]0/[3]0 molar ratio (entries 5–7, Table 1) were also achieved in this case. In contrast to PAz(1), however, a small shoulder progressively appeared in the high molar mass region of SEC traces of PAz(2) (Fig. S2 in the ESI†), as the initial [2]0/[3]0 was increased, i.e. for higher molar masses targeted.
| Run | R | [M]/[3]/[Me5-Ipr] | Time (h) | Conv.a (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) calcd
(g mol−1) calcd
(g mol−1) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp
(g mol−1) exp
(g mol−1) |
Đ |
|---|---|---|---|---|---|---|---|
a Determined by 1H NMR.
b Theoretical molar masses: Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) calcd = ([M]/[3]) × MAz × conv. (MAz = molar mass of one monomer unit).
c Determined by size exclusion chromatography in THF (PS calibration). calcd = ([M]/[3]) × MAz × conv. (MAz = molar mass of one monomer unit).
c Determined by size exclusion chromatography in THF (PS calibration).
|
|||||||
| 1 | Me | 10/1/0.1 | 24 | 100 | 2100 | 1880 | 1.07 |
| 2 | Me | 20/1/0.1 | 24 | 100 | 4200 | 2500 | 1.06 |
| 3 | Me | 50/1/0.1 | 24 | 100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
6000 | 1.06 |
| 4 | Me | 100/1/0.1 | 24 | 100 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.04 |
| 5 | Ph | 20/1/0.1 | 120 | 97 | 5300 | 2600 | 1.06 |
| 6 | Ph | 50/1/0.1 | 120 | 98 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
6250 | 1.12 |
| 7 | Ph | 100/1/0.1 | 120 | 99 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.15 |
The discrepancy noted between experimental and theoretical molar masses (Table 1) was attributed to the calibration of SEC with PS standards. Molar masses of PAz(2), as determined using 1H NMR spectroscopy (5200 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, entries 5 and 7, Table 1, respectively) indeed closely matched theoretical values. Fig. 1a shows a typical 1H NMR of a purified PAz(2) (entry 5), with the presence, in particular, of the characteristic signals of the hexyl group from the amine initiator at 0.7–1.3 ppm. The relative integration of these signals with those corresponding to the main chain protons (d) of PAz(2) allowed estimation of the PAz(2) molar mass (Table 1). The agreement between experimental and theoretical molar masses attested to the complete initiation of polymer chains by secondary amine 3. In the case of PAz(1), overlapping of both signals due to aliphatic protons and to the pendant methyl group in the backbone precluded an accurate determination of the molar masses of these compounds (Fig. S7 in the ESI†).
000 g mol−1, entries 5 and 7, Table 1, respectively) indeed closely matched theoretical values. Fig. 1a shows a typical 1H NMR of a purified PAz(2) (entry 5), with the presence, in particular, of the characteristic signals of the hexyl group from the amine initiator at 0.7–1.3 ppm. The relative integration of these signals with those corresponding to the main chain protons (d) of PAz(2) allowed estimation of the PAz(2) molar mass (Table 1). The agreement between experimental and theoretical molar masses attested to the complete initiation of polymer chains by secondary amine 3. In the case of PAz(1), overlapping of both signals due to aliphatic protons and to the pendant methyl group in the backbone precluded an accurate determination of the molar masses of these compounds (Fig. S7 in the ESI†).
 | ||
| Fig. 1 (a) 1H NMR spectrum of PAz(2) (entry 5, Table 1). Experimental (b) and computed (c) MALDI ToF MS of PAz(1) (entry 1, Table 1). | ||
The presence of the N-hexyl-p-toluenesulfonylamino group from 3 in the α-position could yet be evidenced by MALDI-ToF MS analysis of low molar mass PAz(1) (entry 1, Table 1). A single population, corresponding to cationized PAz(1), with a peak-to-peak mass increment of 211.3 g mol−1 corresponding to the molar mass of one monomer unit derived from 1, was indeed unambiguously observed, as shown in Fig. 1b and c.
In combination, characterization by SEC, NMR and MALDI-ToF MS is consistent with a controlled OROP of N-tosyl aziridines. This was also supported by the perfectly linear evolution of ln([M]0/[M]) vs. time, when monitoring the Me5-Ipr-OROP of 2 using real-time 1H NMR spectroscopy (Fig. S9 and S10, ESI†).
Based on the background literature regarding other examples of NHC-mediated OROP,3–5 either a nucleophilic or a basic mechanism, i.e. through the aziridine or the amine activation by the Me5-Ipr NHC, respectively (vide infra) could be operative (see Scheme S1 in the ESI†). The former mechanism would involve ring opening of the aziridine by direct attack by Me5-Ipr, forming an imidazolium amide zwitterionic intermediate that would be further displaced by the secondary amine (3). However, given the high basicity of Me5-Ipr (pKa = 24 in DMSO13), in particular compared to that of the sulfonylamine group (pKa ≈ 16 in DMSO14), a basic mechansim where the amine initiator/chain end would be deprotonated by Me5-Ipr cannot be ruled out. Data are lacking at this stage to state the correct mechanism, and both experimental and theoretical investigations on these NHC-mediated OROP's of N-sulfonylaziridines are in progress.
In order to verify that a majority of Me5-Ipr-OROP-derived PAz chains could be reactivated, chain extension experiments were performed as follows (see also Fig. S3, ESI†). Polymerization in THF at 50 °C of 20 eq. of 1, using 10 mol% of Me5-Ipr, afforded a PAz(1) precursor (Mn = 2500 g mol−1; Đ = 1.06). Addition of 30 eq. of 1 increased the molar mass to Mn = 6000 g mol−1 while maintaining a low dispersity (Đ = 1.06) after 24 h of the reaction at 50 °C (Fig. S3, ESI†), confirming quantitative reinitiation of the OROP from the PAz(1) precursor. These results prompted us to further investigate the synthesis of PAz-based block copolymers, via a sequential NHC-OROP of 1 and 2. As displayed in Scheme 2, both monomers were added either in this order (1 then 2) or in the other order (2 then 1).
 | ||
| Scheme 2 Synthesis of PAz(1)-b-PAz(2) and PAz(2)-b-PAz(1) block copolymers by sequential OROP catalyzed by Me5-Ipr. | ||
Synthesis of a well-defined PAz(1)-b-PAz(2) block copolymer (8b) was first achieved, i.e. using the more reactive aziridine (1) first (20 eq.), giving a PAz(1) precursor (8a, Mn = 2500 g mol−1; Đ = 1.06; Scheme 2) after 1 day, followed by addition of 20 eq. of the less reactive aziridine (2). Block copolymerization proved effective, as illustrated by the SEC trace of the compound collected after 5 days of reaction at 50 °C in THF, showing a clear shift to the higher molar masses (Mn = 4500 g mol−1; Đ = 1.06) compared with the SEC trace of the PAz(1) macroinitiator (Fig. 2a), Scheme 2, thus attesting to an effective crossover reaction. Interestingly, the PAz(1)-b-PAz(2) block copolymer (9b) could also be obtained by reversing the order of addition of the two monomers (2 then 1; see Scheme 2). A shoulder in the high molar mass region was observed though, presumably to some coupling reactions at high monomer conversions, as shown in Fig. 2b.
 | ||
| Fig. 2 SEC traces (refractometric detection) of (a) PAz(1)-b-PAz(2) and (b) PAz(2)-b-PAz(1) block copolymers (see Scheme 2). | ||
In summary, we describe for the first time the “controlled/living” organocatalyzed ring-opening polymerization (OROP) of N-activated aziridines by an N-heterocyclic carbene (NHC), namely, 1,3-bis(isopropyl)-4,5(dimethyl)imidazol-2-ylidene. Reactions can be conducted in THF at 50 °C in the presence of an N-activated secondary amine as an initiator to mimic the growing chains. This provides a straightforward access to structurally well-defined and metal-free polyaziridines (PAz) that can serve as precursors of linear 2-n-alkyl-substituted poly(ethylene imine)s after removal of the N-tosyl groups. This method can also be applied to achieve well-defined all PAz-based block copolymers by sequential OROP utilizing a carbene catalysis. Overall, this work further expands the monomer substrate diversity in NHC-mediated polymerization reactions.
The authors are grateful to the French Ministry of Education and Research for financial support of CBH and to the European Commission through project SUSPOL-EJD 642671.
Notes and references
- For a review on NHCs, see:
(a) D. Bourissou, O. Guerret, F. P. Gabbai and G. Bertrand, Chem. Rev., 2000, 100, 39–92 CrossRef CAS PubMed
; (b) M. N. Hopkinson and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed
.
- For a review on organocatalysis using NHCs, see:
(a) N. Marion, S. Diez-Gonzalez and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS PubMed
; (b) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed
.
- For a review on organocatalyzed polymerization, see:
(a) M. K. Kiesewetter, E. J. Shin, J. L. Hedrick and R. M. Waymouth, Macromolecules, 2010, 2093–2107 CrossRef CAS
; (b) W. N. Ottou, H. Sardon, D. Mercerreyes, J. Vignolle and D. Taton, Prog. Polym. Sci., 2016, 56, 64–115 CrossRef CAS
.
- For a review on NHC-catalyzed polymerization, see:
(a) M. Fèvre, J. Pinaud, Y. Gnanou, J. Vignolle and D. Taton, Chem. Soc. Rev., 2013, 42, 2142–2172 RSC
; (b) S. Naumann and A. P. Dove, Polym. Chem., 2015, 6, 3185–3200 RSC
.
-
(a) N. E. Kamber, W. Jeong, S. Gonzalez, J. L. Hedrick and R. M. Waymouth, Macromolecules, 2009, 42, 1634–1639 CrossRef CAS
; (b) W. Jeong, E. J. Shin, D. A. Culkin, J. L. Hedrick and R. M. Waymouth, J. Am. Chem. Soc., 2009, 131, 4884–4891 CrossRef CAS PubMed
; (c) J. Raynaud, C. Absalon, Y. Gnanou and D. Taton, J. Am. Chem. Soc., 2009, 131, 3201–3209 CrossRef CAS PubMed
.
-
(a) J. Raynaud, A. Ciolino, A. Baceiredo, M. Destarac, F. Bonnette, T. Kato, Y. Gnanou and D. Taton, Angew. Chem., Int. Ed., 2008, 47, 5390–5393 CrossRef CAS PubMed
; (b) M. D. Scholten, J. L. Hedrick and R. M. Waymouth, Macromolecules, 2008, 41, 7399–7404 CrossRef CAS
.
-
(a) B. Bantu, G. M. Pawar, U. Decker, K. Wurst, A. M. Schmidt and M. R. Buchmeiser, Chem. – Eur. J., 2009, 15, 3103–3109 CrossRef CAS PubMed
; (b) H. Sardon, A. Pascual, D. Mecerreyes, D. Taton, H. Cramail and J. L. Hedrick, Macromolecules, 2015, 48, 3153–31657 CrossRef CAS
.
-
(a) H. A Brown and R. M. Waymouth, Acc. Chem. Res., 2013, 46, 2585–2596 CrossRef PubMed
; (b) S. Naumann, F. G. Schmidt, W. Frey and M. R. Buchmeiser, Polym. Chem., 2013, 4, 4172 RSC
; (c) S. Naumann, F. G. Schmidt, M. Speiser, M. Bohl, S. Epple, C. Bonten and M. R. Buchmeiser, Macromolecules, 2013, 46, 8426–8433 CrossRef CAS
; (d) L. Guo, S. H. Lahasky, K. Ghale and D. Zhang, J. Am. Chem. Soc., 2012, 134, 9163–9171 CrossRef CAS PubMed
.
-
(a) Y. Zhang, M. Schmitt, L. Falivene, L. Caporaso, L. Cavallo and E. Y. Chen, J. Am. Chem. Soc., 2013, 135, 17925–17942 CrossRef CAS PubMed
; (b) M. Hong and E. Y. Chen, Angew. Chem., Int. Ed. Engl., 2014, 53, 11900–11906 CrossRef CAS PubMed
; (c) M. Hong, X. Tang, L. Falivene, L. Caporaso, L. Cavallo and E. Y. Chen, J. Am. Chem. Soc., 2016, 138, 2021–2035 CrossRef CAS PubMed
.
- I. C. Stewart, C. C. Lee, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 17616–17617 CrossRef CAS PubMed
.
- S. C. Bergmeier and P. P. Seth, Tetrahedron Lett., 1999, 40, 6181–6184 CrossRef CAS
.
-
(a) L. Thomi and F. R. Wurm, Macromol. Symp., 2015, 349, 51–56 CrossRef CAS
; (b) E. Rieger, A. Alkan, A. Manhart, M. Wagner and F. R. Wurm, Macromol. Rapid Commun., 2016, 37, 833–839 CrossRef CAS PubMed
; (c) E. Rieger, A. Manhart and F. R. Wurm, ACS Macro Lett., 2016, 5, 195–198 CrossRef CAS
.
- R. W. Alder, P. R. Allen and S. J. Williams, J. Chem. Soc., Chem. Commun., 1995, 1267–1268 RSC
.
- F. G. Bordwell, J. Org. Chem., 1990, 55, 3330–3336 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc04323b |
| This journal is © The Royal Society of Chemistry 2016 |