Palladium-catalyzed dearomatizing 2,5-alkoxyarylation of furan rings: diastereospecific access to spirooxindoles†
Abstract
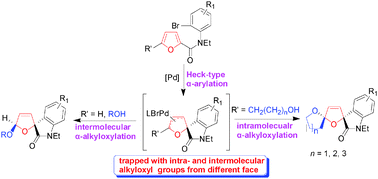
A protocol for Pd-catalyzed intra- and intermolecular 2,5-alkoxyarylation reactions of furans to diastereospecifically synthesize two series of spirooxindoles is reported. This protocol likely involves an intramolecular dearomatizing Heck-type α-arylation of the furan ring to produce a cyclic allylic palladium and the subsequent intra- or intermolecular introduction of an alkyloxyl group at the other α-position of the ring, with different facial selectivities.

 Please wait while we load your content...
Please wait while we load your content...