 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of pristine closo-[Ge10]2−†
Manuel M.
Bentlohner
,
Christina
Fischer
and
Thomas F.
Fässler
 *
*
Technische Universität München, Department Chemie, Lichtenbergstrasse 4, 85747 Garching, Germany. E-mail: Thomas.faessler@lrz.tum.de; Fax: +49 89 289 13186; Tel: +49 89 289 13131
First published on 12th July 2016
Abstract
The first [Ge10]2− Zintl anion, which is neither filled nor connected to another metal atom is presented in terms of X-ray structure, Raman-spectrum and ESI-MS. Pure [Ge10]2−, adapting a D4d symmetric closo-structure, were crystallized from a Rb4Ge9/ethylendiamine solution, containing 7-amino-1-trimethylsilyl-5-aza-hepta-3-en-1-yne. The role of the latter on the formation of [Rb(222-crypt)]2[Ge10](en)1.5 is discussed.
The soft oxidation of nido-[E9]4− Zintl anions (E = Ge, Sn, Pb) with 22 skeleton electrons (SE) is a powerful method for the synthesis of new types of the heavier representatives of group 14 clusters and led to a large variety of cage-like structures.1–5 By that strategy new element allotropes4–5 as well as ordered, (nano)porous forms of germanium have been obtained.6–8 Although a comprehensive understanding of the cluster oxidation and thus a control over the reaction outcome is still lacking, a large number of investigations on the oxidation of [E9]4− clusters in solution has been performed during the last couple of years,1,2 and a broad variety of coupled clusters {[(Ge9)m]}q− (m = 2–4, ∞) has been obtained by soft oxidation of [Ge9]4− in ethylenediamine (en), N,N-dimethylformamide (dmf) and liquid ammonia. Even though in most cases the reactions are not understood in detail,9–16 mild oxidative properties have been ascribed to the involved solvents,5,17–19 and recently we have shown that the solvent en indeed plays an important role in the cluster formation.8
It has been found that oxidative reaction conditions not only can trigger the coupling but also the growth of clusters.20 Theoretical investigations showed that for E = Ge a full oxidation to novel germanium allotropes under retention of the polyhedral structure is reasonable.21 The reaction of [E9]4− with organometallic complexes MLa (M = metal, L = ligand) in en, dmf and liquid ammonia yielded a broad variety of endohedrally filled clusters [M@En]q− (n ≥ 9),1–3 which in special cases adapt non-deltahedral structures and transition metal complexes of clusters with up to 45 covalently connected Ge atoms.22–25 The formation of [M@En]q− (n > 9), from [E9]4− cages, highlights the ability of these tetrel clusters to structurally reorganize in solution.26,27
The Zintl anions [Pb10]2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 and [(Ge10)Mn(CO)4]3−
28 and [(Ge10)Mn(CO)4]3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 are scarce examples of empty homoatomic ten-vertex tetrel clusters, and recently we extended the series of structurally characterized heteroatomic correspondents.26,30,31 In [Ge9SnGe9]4− a formally closo-[Ge9Sn]2− unit coordinates to a [Ge9]2− cluster.32 In case of [M@En]q− a stabilizing effect of the interstitial M atom on the surrounding [En] cage has been evidenced by quantum-chemical calculations, indicating the preferred formation of endohedrally filled clusters with n > 9 instead of their empty correspondents.1–3,20
29 are scarce examples of empty homoatomic ten-vertex tetrel clusters, and recently we extended the series of structurally characterized heteroatomic correspondents.26,30,31 In [Ge9SnGe9]4− a formally closo-[Ge9Sn]2− unit coordinates to a [Ge9]2− cluster.32 In case of [M@En]q− a stabilizing effect of the interstitial M atom on the surrounding [En] cage has been evidenced by quantum-chemical calculations, indicating the preferred formation of endohedrally filled clusters with n > 9 instead of their empty correspondents.1–3,20
The formation of the empty pristine [Pb10]2− unit on the one hand and of [(Ge10)Mn(CO)4]3− on the other also suggests the existence of an unbound [Ge10]2− Zintl anion. An earlier report on such a [Ge10]2− cluster33 turned out to be rather questionable because a disordered closo-[Ge9]2− cluster (Fig. S1, ESI†) was unequivocally characterized in similar crystals.‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 Although the isolation of crystals containing the unbound and empty [Ge10]2− Zintl anion has been unsuccessful so far, the latter is a frequently observed species in mass spectra obtained by laser desorption experiments or from solutions of Zintl phases in polar organic solvents.29,32,35,36
34 Although the isolation of crystals containing the unbound and empty [Ge10]2− Zintl anion has been unsuccessful so far, the latter is a frequently observed species in mass spectra obtained by laser desorption experiments or from solutions of Zintl phases in polar organic solvents.29,32,35,36
Herein we report on the synthesis and characterization of [Rb(222-crypt)]2[Ge10](en)1.5 (1) which contains such an empty and unbound [Ge10]2− Zintl anion. Compound 1 was characterized by single crystal X-ray structure analysis, Raman-spectroscopy and electrospray ionization mass spectrometry (ESI-MS). Further, we present an ESI-MS investigation on the involved reaction solutions in order to shed some light on the formation of 1.
Dark purple pillars of 1 were obtained (yield ca. 10–20%) from a solution of Rb4Ge9 (1 eq.) and 7-amino-1-trimethylsilyl-5-aza-hepta-3-en-1-yne (1 eq.)37 in en after layering of the solution with toluene/cryptand[2.2.2] (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane; for experimental details see ESI†).
Crystals of 1 (Fig. S2, ESI†) contain two [Rb(222-crypt)]+ cations per cluster unit, and thus a formal charge of −2 can be assigned to the anionic cluster entity (Fig. 1a). [Ge10]2− (1a) consists of ten symmetry-independent germanium atoms and adapts the shape of a bi-capped square antiprism. The atoms of the planes A (Ge2 to Ge5) and B (Ge6 to Ge9) are nearly perfect squares with ratios of the face diagonals of 1.01 and 1.00 and torsion angles of 179.8° and 179.9°, respectively. The side lengths of A and B are in the narrow ranges of 2.760(1) Å (Ge2–Ge3) to 2.799(1) Å (Ge4–Ge5) and 2.780(1) Å (Ge7–Ge8) to 2.822(1) Å (Ge6–Ge9). Moreover, similar inter-square Ge–Ge distances from 2.535(1) Å (Ge3–Ge7) to 2.566(1) Å (Ge4–Ge9) indicate that A and B are in parallel. The mean inter-square Ge–Ge distance d1(1a) = 2.55(1) Å is considerably shorter than the mean Ge–Ge distances within A and B [d2(1a) = 2.79(2) Å, d2′(1a) = 2.80(2) Å]. The two atoms Ge1 and Ge10 cap the quadratic antiprism, whereby d3(1a) = 2.583(7) Å and d3′(1a) = 2.59(2) Å are slightly longer than d1(1a) = 2.55(1) Å. In summary 1a adopts a nearly perfect D4d symmetry.
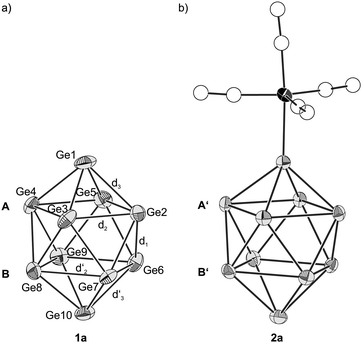 | ||
| Fig. 1 (a) closo-[Ge10]2− (1a) and (b) [(Ge10)Mn(CO)4]3− (2a)29 for comparison. Square planes of 1a and 2a are labeled with A/B and A′/B′, respectively. (a and b) Ge and Mn atoms are shown as grey and black ellipsoids, respectively, at a probability level of 50%. C and O atoms are shown as empty spheres. | ||
The geometrical parameters of 1a are very similar to those of [(Ge10)Mn(CO)4]3− (2a) (Fig. 1b). Like for 1a, the [Ge10] cluster in 2a adapts D4d symmetry. The mean Ge–Ge distances d3(2a) and d3′(2a) are both 2.58(1) Å, suggesting that d3(2a) is not influenced by the coordination of the Mn(CO)4 fragment. However, in contrast to the square planes in 1a, A′ is significantly widened [d2(2a) = 2.85(2) Å] compared to B′ [d2′(2a) = 2.77(1) Å], which might be attributed to the neighboring Mn(CO)4 fragment. The inter-square Ge–Ge distances are almost identical for 1a and 2a [d1(1a) = 2.55(1) Å, d1(2a) = 2.547(8) Å].29
According to Wade's rules, 1a can be described as a closo-deltahedron with 22 skeleton electrons (SE), whereby each vertex atom contributes two electrons, plus two extra electrons due to the two-fold negative charge.38
In order to study the vibrational behavior of 1a, single crystals of 1 were investigated by Raman spectroscopy. The spectrum (Fig. 2a) shows a very strong signal at 209 cm−1 and several very weak bands in the range from 95 to 166 cm−1. In comparison, the Raman spectrum of the compound [K(222-crypt)]2[Ge9] exhibits one very intensive peak at 212 cm−1 and three signals below 200 cm−1 of medium intensity. Quantum-chemical calculations showed that the most intensive mode at 212 cm−1 corresponds to the “breathing” of the closo-[Ge9]2− cluster. At least one of the medium intensive signals is attributed to vibrations of the central trigonal prism.34 For nido-[Ge9]4− clusters (Fig. 2b) the “breathing” mode appears at higher wavenumbers of ca. 222 cm−1, and below 150 cm−1 medium-intensive signals are visible.39–41 However, the latter appear in a neat solid with stronger alkaline metal–Ge interactions. In the spectrum of 1 the absence of intensive signals below 200 cm−1 evidences, that 1 does not contain [Ge9]2− clusters, and thus we conclude that the mode at 209 cm−1 corresponds to the “breathing” vibration of 1a.34,39–41
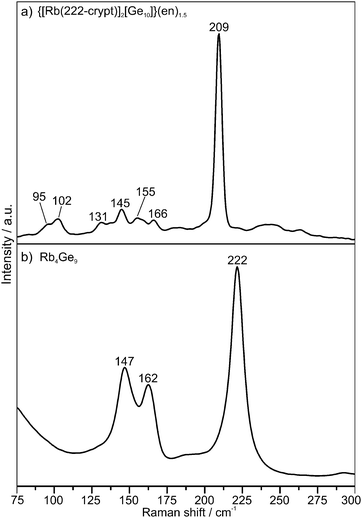 | ||
| Fig. 2 Raman spectrum of (a) 1 and (b) Rb4Ge9. Characteristic modes are labeled with the corresponding Raman shifts. | ||
Crystals of 1 were obtained only from Rb4Ge9/en mixtures in the presence of 7-amino-1-trimethylsilyl-5-aza-hepta-3-en-1-yne (3), but not in the absence of 3. Therefore we investigated several solutions by ESI-MS, namely 1 in acetonitrile (acn) (Fig. S3, ESI†) as well as Rb4Ge9/en and Rb4Ge9/en/3 with a molar ratio Rb4Ge9/3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at an equal concentration of Rb4Ge9 in en for both mixtures (Fig. S4, ESI†).
1 at an equal concentration of Rb4Ge9 in en for both mixtures (Fig. S4, ESI†).
Crystals of 1 readily dissolve in acn (denoted as 1/acn) giving a deep brown solution. Immediate injection of this solution into the mass spectrometer leads to peaks indicative for the presence of Ge10− (m/z = 725), {Ge10Rb}− (m/z = 812), and {Ge10Rb(222-crypt)}− (m/z = 1188), with the latter one as the most prominent species. The occurrence of solely Ge10 units hints for an enhanced stability of this cluster. By contrast, the ESI-MS of Rb4Ge9/en (Fig. S4a, ESI†) reveals the presence of {HxGe9}− (x = 0–2; m/z = 653, 654, 655), {HGe10}− (m/z = 726) and {Ge9Rb}− (m/z = 738) with an approximate ratio of intensities of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The high abundance of {HGe10}− indicates that 1a is readily formed upon solution of Rb4Ge9 in en, by a not yet understood fragmentation of the original [Ge9]4− cluster.§¶
1. The high abundance of {HGe10}− indicates that 1a is readily formed upon solution of Rb4Ge9 in en, by a not yet understood fragmentation of the original [Ge9]4− cluster.§¶
Interestingly, the mass spectrum of the solution of Rb4Ge9/3/en (Fig. S4b, ESI†), from which the crystals of 1a were obtained, shows dominant signals of {Ge9R}− (m/z = 764), {Ge8R}− (m/z = 692) and {Ge7R}− (m/z = 618) (R = 7-amino-5-aza-hepta-2,4-dien-2-yl) as well as the non-alkenylated species {HxGe9}− (x = 0–2), {Ge9Rb}−||![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 and {HGe10}−. The high abundance of clusters bearing organic ligands R, that arise from the nucleophilic addition of one and two molecules of 3 to the [Ge9]4− unit, documents the higher reactivity of the [Ge9]4− unit compared to that of [Ge10]2−.37,42 The appearance of {HGe10}− suggests that a fraction of the initial [Ge9]4− clusters reacts to 1a prior to the reaction with 3. Thus, layering of a Rb4Ge9/3/en solution with cryptand[2.2.2] in toluene preferably produces crystals of 1 since the functionalized species [Ge9R]3− obviously do not crystalize under these conditions. The binding mode of the organic group R to the cluster is shown in Fig. S5 (ESI†).
42 and {HGe10}−. The high abundance of clusters bearing organic ligands R, that arise from the nucleophilic addition of one and two molecules of 3 to the [Ge9]4− unit, documents the higher reactivity of the [Ge9]4− unit compared to that of [Ge10]2−.37,42 The appearance of {HGe10}− suggests that a fraction of the initial [Ge9]4− clusters reacts to 1a prior to the reaction with 3. Thus, layering of a Rb4Ge9/3/en solution with cryptand[2.2.2] in toluene preferably produces crystals of 1 since the functionalized species [Ge9R]3− obviously do not crystalize under these conditions. The binding mode of the organic group R to the cluster is shown in Fig. S5 (ESI†).
Our investigations shed some light onto the formation of the [Ge10]2− Zintl anion. ESI-MS investigations revealed that the [Ge10]2− unit is readily formed upon simple dissolution of Rb4Ge9 in en, highlighting the flexibility of the dissolved tetrel element [Ge9]4− clusters which can grow and thereby change their shape. It turned out that the crystallization of the bare [Ge9]y− (y = 2–4) clusters is favored over the crystallization of [Ge10]2−, both of which are present in Rb4Ge9/en solutions. Obviously, the [Ge10]2− unit can only be obtained when the Ge9 clusters are “masked” by the reaction with 7-amino-1-trimethylsilyl-5-aza-hepta-3-en-1-yne, leading to [RGe9]3−, which remains in solution and does not crystalize by layering with cryptand[2.2.2] in toluene. By adjusting the experimental conditions, it might be possible to obtain even larger empty germanium cages, and it also is feasible that other representatives of the [E10]2− series can be synthesized by this method.
The authors are grateful to the SolTech (Solar Technologies go Hybrid) program of the State of Bavaria for financial support. Moreover, the authors thank Herta Slavik for Raman-spectroscopic measurements and Dr Wilhelm Klein for the help with the crystal structure analysis.
Notes and references
- T. F. Fässler and S. D. Hoffmann, Angew. Chem., Int. Ed., 2004, 43, 6242 CrossRef PubMed.
- S. C. Sevov and J. M. Goicoechea, Organometallics, 2006, 25, 5678 CrossRef CAS.
- S. Scharfe, F. Kraus, S. Stegmaier, A. Schier and T. F. Fässler, Angew. Chem., Int. Ed., 2011, 50, 3630–3670 CrossRef CAS PubMed.
- A. M. Guloy, R. Ramlau, Z. Tang, W. Schnelle, M. Baitinger and Y. Grin, Nature, 2006, 443, 320 CrossRef CAS PubMed.
- T. F. Fässler, Angew. Chem., Int. Ed., 2007, 46, 2572 CrossRef PubMed.
- D. Sun, A. E. Riley, A. J. Cadby, E. K. Richman, S. D. Korlann and S. H. Tolbert, Nature, 2006, 441, 1126 CrossRef CAS PubMed.
- G. S. Armatas and M. G. Kanatzidis, Science, 2006, 313, 817 CrossRef CAS PubMed.
- M. M. Bentlohner, M. Waibel, P. Zeller, K. Sarkar, P. Müller-Buschbaum, D. Fattakhova-Rohlfing and T. F. Fässler, Angew. Chem., Int. Ed., 2016, 55, 2441 CrossRef CAS PubMed.
- L. Xu and S. C. Sevov, J. Am. Chem. Soc., 1999, 121, 9245 CrossRef CAS.
- C. Downie, Z. J. Tang and A. M. Guloy, Angew. Chem., Int. Ed., 2000, 39, 337 CrossRef.
- A. Ugrinov and S. C. Sevov, J. Am. Chem. Soc., 2002, 124, 10990 CrossRef CAS PubMed.
- R. Hauptmann and T. F. Fässler, Z. Anorg. Allg. Chem., 2003, 629, 2266 CrossRef CAS.
- A. Ugrinov and S. C. Sevov, Inorg. Chem., 2003, 42, 5789 CrossRef CAS PubMed.
- L. Yong, S. D. Hoffmann and T. F. Fässler, Z. Anorg. Allg. Chem., 2004, 630, 1977 CrossRef CAS.
- A. Nienhaus, S. D. Hoffmann and T. F. Fässler, Z. Anorg. Allg. Chem., 2006, 632, 1752 CrossRef CAS.
- S. Scharfe and T. F. Fässler, Z. Anorg. Allg. Chem., 2011, 637, 901 CrossRef CAS.
- A. Ugrinov and S. C. Sevov, J. Am. Chem. Soc., 2003, 125, 14059 CrossRef CAS PubMed.
- A. Ugrinov and S. C. Sevov, Chem. – Eur. J., 2004, 10, 3727 CrossRef CAS PubMed.
- C. Downie, J. G. Mao, H. Parmar and A. M. Guloy, Inorg. Chem., 2004, 43, 1992 CrossRef CAS PubMed.
- E. N. Esenturk, J. Fettinger, Y.-F. Lam and B. Eichhorn, Angew. Chem., Int. Ed., 2004, 43, 2132 CrossRef CAS PubMed.
- A. J. Karttunen, T. F. Fässler, M. Linnolahti and T. A. Pakkanen, ChemPhysChem, 2010, 11, 1944 CAS.
- A. Spiekermann, S. D. Hoffmann, T. F. Fässler, I. Krossing and U. Preiss, Angew. Chem., Int. Ed., 2007, 46, 5310 CrossRef CAS PubMed.
- J.-Q. Wang, S. Stegmaier and T. F. Fässler, Angew. Chem., Int. Ed., 2009, 48, 1998 CrossRef CAS PubMed.
- B. Zhou, M. S. Denning, D. L. Kays and J. M. Goicoechea, J. Am. Chem. Soc., 2009, 131, 2802 CrossRef CAS PubMed.
- G. Espinoza-Quintero, J. C. A. Duckworth, W. K. Myers, J. E. McGrady and J. M. Goicoechea, J. Am. Chem. Soc., 2014, 136, 1210 CrossRef CAS PubMed.
- M. M. Gillett-Kunnath, I. Petrov and S. C. Sevov, Inorg. Chem., 2010, 49, 721 CrossRef CAS PubMed.
- M. M. Gillett-Kunnath, A. G. Oliver and S. C. Sevov, J. Am. Chem. Soc., 2011, 133, 6560 CrossRef CAS PubMed.
- A. Spiekermann, S. D. Hoffmann and T. F. Fässler, Angew. Chem., Int. Ed., 2006, 45, 3459 CrossRef CAS PubMed.
- D. Rios and S. C. Sevov, Inorg. Chem., 2010, 49, 6396 CrossRef CAS PubMed.
- M. Waibel and T. F. Fässler, Inorg. Chem., 2013, 52, 5861 CrossRef CAS PubMed.
- D. Rios, M. M. Gillett-Kunnath, J. D. Taylor, A. G. Oliver and S. C. Sevov, Inorg. Chem., 2011, 50, 2373 CrossRef CAS PubMed.
- M. M. Bentlohner, L.-A. Jantke, T. Henneberger, C. Fischer, K. Mayer, W. Klein and T. F. Fässler, Chem. – Eur. J., 2016 DOI:10.1002/chem.201601706.
- C. Belin, H. Mercier and V. Angilella, New J. Chem., 1991, 15, 931 CAS.
- J. Åkerstedt, S. Ponou, L. Kloo and S. Lidin, Eur. J. Inorg. Chem., 2011, 3999 CrossRef.
- T. F. Fässler, H.-J. Muhr and M. Hunziker, Eur. J. Inorg. Chem., 1998, 1433–1438 CrossRef.
- S. Mitzinger, L. Broeckaert, W. Massa, F. Weigend and S. Dehnen, Nat. Commun., 2016, 7, 10480 CrossRef CAS PubMed.
- M. M. Bentlohner, W. Klein, Z. H. Fard, L.-A. Jantke and T. F. Fässler, Angew. Chem., Int. Ed., 2015, 54, 3748 CrossRef CAS PubMed.
- K. Wade, Inorg. Nucl. Chem. Lett., 1972, 8, 559 CrossRef CAS.
- H. G. Von Schnering, M. Baitinger, U. Bolle, W. Carrillo-Cabrera, J. Curda, Y. Grin, F. Heinemann, J. Llanos, K. Peters, A. Schmeding and M. Somer, Z. Anorg. Allg. Chem., 1997, 623, 1037 CrossRef CAS.
- M. Somer, W. Carrillo-Cabrera, E. M. Peters, K. Peters and H. G. v. Schnering, Z. Anorg. Allg. Chem., 1998, 624, 1915 CrossRef CAS.
- V. Hlukhyy, T. F. Fässler, S. Ponou, S. Lidin, N. P. Ivleva and R. Niessner, Inorg. Chem., 2012, 51, 4058 CrossRef CAS PubMed.
- M. W. Hull and S. C. Sevov, J. Am. Chem. Soc., 2009, 131, 9026 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic details, ESI-MS spectra. CCDC 1479637. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04143d |
| ‡ Both Belin and Akerstedt isolated [K(222-crypt)]2[Ge9], which undergoes a disorder/order transition between 250 K and 100 K. Belin et al. performed single-crystal X-ray structure analysis at 250 K, and described the disordered [Ge9]2− clusters as [Ge10]2−. Akerstedt et al. reinvestigated the same compound (identical unit cell and cell volume) at 100 K, and observed a fully ordered closo-[Ge9]2− cluster.33,34 |
| § The formation of 1a is an oxidative process (Scheme S1, ESI†), as the formal number of valence electrons per Ge atom, reduces from 22/9 in case of [Ge9]4− to 22/10 for 1a. |
| ¶ Layering of such solutions with cryptand[2.2.2] or 18-crown-6 (1,4,7,10,13,16-hexaoxacyclooctadecane) in toluene has yielded a variety of crystals containing (connected) Ge9 clusters, but none comprising 1a.17 |
| || The occurrence of also Ge9− and (Ge9Rb)− in ESI-MS most likely is attributed to the cleavage of the Ge–C bonds of [Ge9R]3− under ESI-MS conditions.42 |
| This journal is © The Royal Society of Chemistry 2016 |
