Open Access Article
Open Access ArticleA BODIPY-based fluorescent probe for ratiometric detection of gold ions: utilization of Z-enynol as the reactive unit†
Muhammed
Üçüncü
,
Erman
Karakuş
and
Mustafa
Emrullahoğlu
*
Department of Chemistry, Faculty of Science, İzmir Institute of Technology, Urla, 35430, Izmir, Turkey. E-mail: mustafaemrullahoglu@iyte.edu.tr
First published on 6th June 2016
Abstract
Using an irreversible intramolecular cyclisation pathway triggered by gold ions, a boron-dipyrromethene (BODIPY) based fluorescent probe integrated with a reactive Z-enynol motif responds selectively to gold ions. With the addition of gold(III), the probe displays ratiometric fluorescence behaviour clearly observable to the naked eye under both visible and UV light.
Though primarily known for its monetary value, gold has recently attracted attention for its impressive catalytic features as well. In synthetic chemistry, the use of gold species has opened new avenues in the synthesis of complex molecular structures.1 However, the increasing use of gold species in chemical synthesis is concomitantly raising hard-to-ignore health and environmental risks due to their potential toxicity. The intake of gold ions in living systems has been widely found to damage vital human organs, including the kidney and liver, as well as the peripheral nervous system.2 It is therefore crucial for the scientific community to be able to assess the levels of gold species in certain chemical, environmental, and biological matrices.
In that context, research continues to focus on developing analytical tools for probing gold species, among which fluorescent-based assays have attracted particular attention for allowing the real-time visualisation of target species in cellular milieus. In recent years, numerous types of fluorescent gold ion probes have appeared in literature on the topic, most of which involve using specific chemical reactions to exploit the alkynophilic nature of gold ions.3 By extension, various fluorescent molecules—rhodamine,4 boron-dipyrromethene (BODIPY),5 fluorescein,6 and naphthalimide7—have been used as signal-reporting fluorophores to recognise gold species by colorimetric or fluorometric changes, if not both, in which optical output is usually recognised as an increase in or activation of emission intensity. However, measurements based on intensity changes are easily influenced by a host of environmental factors, including concentration variations, photo-bleaching, and excitation intensity.
To overcome the barriers generally associated with intensity-based sensors, measuring optical signals as intensity ratios at two different wavelengths seems quite promising, for it would allow for a built-in correction for environmental effects. Until now, examples of fluorescent probes based on the ratiometric detection of gold ions have been inadequate, due to the absence of effective strategies and guidelines for designing ratiometric fluorescent molecules.5a,6a,8b In a recent contribution, we introduced a new strategy for ratiometrically sensing gold ions by exploiting the catalytic behaviour of gold ions to transform a highly conjugated probe structure into a non-conjugated one, by which the presence of gold species became detectable as an emission ratio of two distinct wavelengths.5a However, cross-sensitivity towards mercury(II) ions is still a challenge to be addressed. Other examples of ratiometric gold ion probes available in recent literature are extensions of intensity-based sensors that draw upon a principle of fluorescence resonance energy transfer to achieve ratiometric response.8
It is a well-known fact that the extent of π-conjugation within the fluorophore–chromophore structure exerts a dramatic effect on the frontier orbital energy levels of the molecules. With the conjugation of fluorescent dye the energy difference between the orbitals decreases, which often results in a redshift of absorption and emission wavelengths.
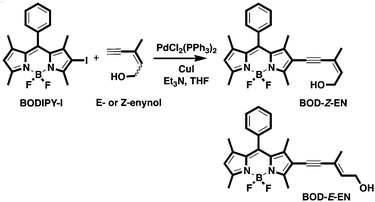
Based on these considerations, we designed and constructed BOD-Z-EN by integrating a Z-enynol motif into a BODIPY-based fluorophore scaffold, with the expectation of generating a highly π-conjugated BODIPY derivative emitting at a wavelength distinctly longer than its unmodified scaffold (Scheme 1). We selected a BODIPY-based fluorophore as the signal reporter for not only its outstanding photo-physical features, but also its easy chemical modification.9 In the presence of gold species, as reported in the literature, substituted Z-enynols can efficiently cyclize to their corresponding furan derivatives.10
We envisioned that an intramolecular cyclisation triggered by gold ions could break the π-conjugation and generate a new BODIPY structure without that conjugation. We thought that the structural modification of the fluorescent probe would eventually yield a significant blue-shift in the fluorophore's emission wavelength, which would thus enable us to recognise the presence of gold species as a ratio of two distinct wave-lengths, by following the chief principle of ratiometric sensing (Scheme 1).
The fluorescent probe BOD-Z-EN was prepared by way of a straightforward synthetic pathway, as outlined in Scheme 2. The monoiodo derivative of BODIPY (BODIPY-I) prepared individually was coupled with Z-enynol [(Z)-3-methylpent-2-en-4-yn-1-ol] according to a Sonogashira coupling protocol in order to induce the desired probe structure in a moderate yield.11 Following chromatographic purification, the identity of the title compound was clearly confirmed by analytical data collected with nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry.12 Using the synthetic route we prepared BOD-E-EN, the E-isomer of the probe, in order to elucidate the mechanism of gold ion detection.
With both compounds in hand, we first investigated the spectral properties of BOD-Z-EN and its response to a range of metal ions in a solution buffered to physiological pH (0.1 M phosphate buffer/EtOH (pH 7.0, v/v, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)).
3)).
We commenced spectral investigation by screening the optical behaviour of the probe in response to the addition of gold ions. In the absence of any analyte species, BOD-Z-EN displayed a single absorption band at 532 nm (Fig. 1a). Meanwhile, in the fluorescence spectrum of BOD-Z-EN, collected upon excitation at 460 nm, a long wavelength emission band at 575 nm was clearly observed. As expected, upon adding AuCl3 (10 equiv.), we observed a new emission band at 512 nm, with a sharp, concomitant decrease in emission band intensity at 575 nm (Fig. 1b).
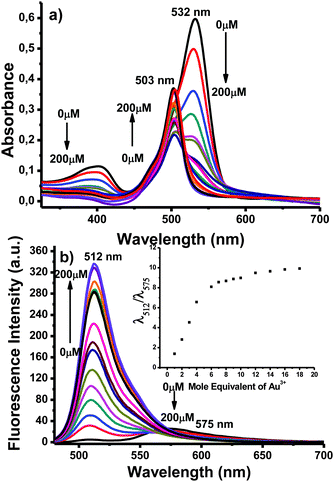 | ||
Fig. 1 (a) Absorbance and (b) fluorescence titration spectra of BOD-Z-EN (10 μM) + Au3+ (1 to 200 μM), 0.1 to 20 equiv.) in 0.1 M phosphate buffer/EtOH (pH 7.0, v/v, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Inset: Calibration curve. 3). Inset: Calibration curve. | ||
The probe's response to AuCl3 was clearly visible to the naked eye; in the presence of gold species, the probe solution's orange emission became distinctly green, which we attributed to a structural modification of the dye structure (Scheme 1). A new green emissive compound, as monitored on a thin-layer chromatography plate, was additional clear evidence of the formation of a non-conjugated core BODIPY derivative (Fig. S11, ESI†). Following chromatographic purification, the structure of the new BODIPY derivative was confirmed by NMR spectroscopy and high-resolution mass spectrometry (ESI-TOF) as BOD-FUR (quantum yield; ΦF = 0.90), the intramolecular cyclisation product of BOD-Z-EN (ΦF = 0.05).12
Our investigation continued with the systematic addition of gold ions to the probe solution. Upon adding Au3+ (0–10 equiv.), we observed the absorption band at 532 nm decrease gradually, with a concomitant linear increase of a new absorbance band at 503 nm (Fig. 1a). A similar trend occurred in fluorescence emission behaviour; with an increased concentration of Au3+ across a wide concentration range, fluorescence emission intensity at 512 nm increased linearly (Fig. 1b). The response of BOD-Z-EN to Au3+ (e.g., 1 equiv. of AuCl3) was exceptionally fast (<30 s), and within a couple of minutes, emission intensity (>65-fold) became completely saturated (Fig. S9, ESI†). An 80-fold enhancement in emission ratio occurred when 10 equiv. of gold was introduced. Under optimum sensing conditions, the detection limit of BOD-Z-EN for detecting Au3+ was 293 nM, based on the signal-to-noise ratio (S/N = 3) (Fig. S1, ESI†).
The metal ion selectivity test for BOD-Z-EN resulted in no obvious spectral changes for competing alkynophilic metal species, including Ag+, Ni2+, Pd2+, Hg2+, Cu2+, and other metal species such as Mg2+, Mn2+, Pb2+, Zn2+, Cd2+, Fe3+, K+, Li+, Ba2+, and Ca2+. Only the addition of Au3+ and, to a lesser extent the addition of Au+ resulted in an increase of fluorescence at 512 nm, which obviously implied the high selectivity of BOD-Z-EN to gold ions (Fig. S6, ESI†). Remarkably, other metal ions did not interfere with the detection of gold ions, and the spectral response induced by Au3+ ions remained unaffected in the presence of any of those metal species. Such results indicate that BOD-Z-EN can properly detect Au3+ ions in mixtures with other related species.12
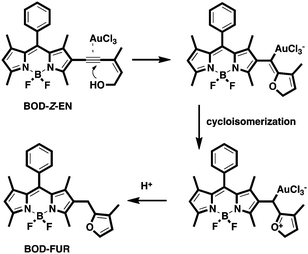
Analogous to reports in the literature,10 we propose that the sensing mechanism occurs when Au3+ activates the triple bond and the subsequent intramolecular exo-dig cyclisation of hydroxyl group to the triple bond, which, in forming a gold intermediate and a rapid isomerisation and protonolysis pathway, affords the BODIPY–furan structure (BOD-FUR) (Scheme 3).
In establishing the most efficient conditions for operation, BOD-Z-EN in the absence of Au3+ was quite stable, even in strong acidic and basic conditions. Likewise, the response of BOD-Z-EN to the addition of Au3+ ions remained unaffected by altering the pH of the sensing media (Fig. S4, ESI†). As such, BOD-Z-EN operates efficiently over a wide pH range (pH 2–12), especially in physiological conditions (pH 7.4), which fulfils a basic requirement for cell bioimaging.
In sharp contrast to BOD-Z-EN, the E-stereoisomer of the probe, BOD-E-EN, was inert to gold, as well as other metal species, despite being in identical sensing conditions. Consistent with the proposed mechanism and in agreement with reports in the literature, adding Au3+ did not alter the emission pattern of BOD-E-EN, thereby indicating the sensing process's high stereoselectivity (Fig. S10, ESI†).
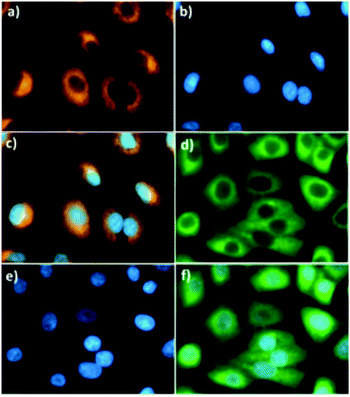
Relying on the promising photochemical and physical properties of BOD-Z-EN, including its rapid response time, unique gold ion specificity, exceptionally low detection limit, and high fold ratiometric change, among other qualities, we next evaluated its potential for tracking Au3+ ions in living cells. To that end, A549 human lung adenocarcinoma cells were incubated at 37 °C first with BOD-Z-EN (5.0 μM) for 20 min, followed by incubation with Au3+ (10 μM) for another 20 min. Using fluorescence microscopy, we could clearly monitor the ratiometric sensing behaviour of the probe toward Au3+ in the cells (Fig. 2). Consistent with results observed in the solution, following incubation with Au3+ the orange emissive cells immediately turned green, thereby unambiguously showing that BOD-Z-EN is cell-membrane permeable and has great potential for use in imaging Au3+ in living cells. Moreover, there were no indications of cell damage. Cells were intact and showed healthy spread and adherent morphology throughout the cell imaging process. The potential cytotoxicity of the probe was evaluated using a standard MTT assay with A549 cell line. The cellular viability was estimated to be 100% after 24 h, which showed that the probe (<5 μM) has no cytotoxicity (Fig. S2, ESI†).
In sum, we devised a fluorescent probe that shows a ratiometric fluorescence response to gold ion species with high sensitivity and selectivity over other metal ion species. The fluorescent probe is built upon a BODIPY fluorophore scaffold that uses a highly novel reactive unit (e.g., Z-enynol) for Au3+ ions. After structural modification triggered by gold ions—namely, an intramolecular cyclisation—the high wavelength-emitting conjugated probe structure transformed into a non-conjugated, low wavelength-emitting structure, thereby allowing us to recognise gold ions as a ratio of distinct emission wavelengths. Apart from the rapid (<30 s), sensitive (293 nM), and highly specific response to gold species in the solution, the probe proved highly successful in imaging gold ions in living cells.
We thank Izmir Institute of Technology (IZTECH) and TUBTAK (113Z601) for financial support and Izmir Institute of Technology, Biotechnology and Bioengineering Research and Application Centre for fluorescence imaging facilities.
Notes and references
- (a) B. Alcaide and P. Almendros, Acc. Chem. Res., 2014, 47, 939–952 CrossRef CAS PubMed; (b) I. Braun, A. M. Asiri and A. S. K. Hashmi, ACS Catal., 2013, 3, 1902–1907 CrossRef CAS; (c) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994–2009 CrossRef CAS PubMed; (d) A. Arcadi, Chem. Rev., 2008, 108, 3266–3325 CrossRef CAS PubMed; (e) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766–1775 RSC; (f) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239–3265 CrossRef CAS PubMed.
- (a) C. M. Goodman, C. D. McCusker, T. Yilmaz and V. M. Rotello, Bioconjugate Chem., 2004, 15, 897–900 CrossRef CAS PubMed; (b) A. Habib and M. Tabata, J. Inorg. Biochem., 2004, 98, 1696–1702 CrossRef CAS PubMed; (c) W. D. Block and E. L. Knapp, J. Pharmacol. Exp. Ther., 1945, 83, 275–278 CAS.
- (a) S. Singha, D. Kim, H. Seo, S. W. Cho and K. H. Ahn, Chem. Soc. Rev., 2015, 44, 4367–4399 RSC; (b) J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- (a) M. Emrullahoğlu, E. Karakuş and M. Üçüncü, Analyst, 2013, 138, 3638–3641 RSC; (b) L. Yuan, W. Lin, Y. Yang and J. Song, Chem. Commun., 2011, 47, 4703–4705 RSC; (c) O. A. Egorova, H. Seo, A. Chatterjee and K. H. Ahn, Org. Lett., 2010, 12, 401–403 CrossRef CAS PubMed; (d) M. J. Jou, X. Chen, K. M. K. Swamy, H. N. Kim, H.-J. Kim, S. G. Lee and J. Yoon, Chem. Commun., 2009, 7218–7220 CAS; (e) Y. K. Yang, S. Lee and J. Tae, Org. Lett., 2009, 11, 5610–5613 CrossRef CAS PubMed.
- (a) M. Üçüncü, E. Karakuş and M. Emrullahoğlu, Chem. – Eur. J., 2015, 21, 13201–13205 CrossRef PubMed; (b) C. Cantürk, M. Üçüncü and M. Emrullahoğlu, RSC Adv., 2015, 5, 30522–30525 RSC; (c) E. Karakuş, M. Üçüncü and M. Emrullahoğlu, Chem. Commun., 2014, 50, 1119–1121 RSC; (d) M. Üçüncü and M. Emrullahoğlu, Chem. Commun., 2014, 50, 5884–5886 RSC; (e) J.-B. Wang, Q.-Q. Wu, Y.-Z. Min, Y.-Z. Liu and Q.-H. Song, Chem. Commun., 2012, 48, 744–746 RSC; (f) M. Üçüncü, E. Karakuş and M. Emrullahoğlu, New J. Chem., 2015, 39, 8337–8341 RSC; (g) E. Karakuş, G. Cakan-Akdogan and M. Emrullahoğlu, Anal. Methods, 2015, 7, 8004–8008 RSC.
- (a) H. Seo, M. E. Jun, O. A. Egorova, K. H. Lee, K. T. Kim and K. H. Ahn, Org. Lett., 2012, 14, 5062–5065 CrossRef CAS PubMed; (b) N. Y. Patil, V. S. Shinde, M. S. Thakare, P. H. Kumar, P. R. Bangal, A. K. Barui and C. R. Patra, Chem. Commun., 2012, 48, 11229–11231 RSC; (c) S. Kambam, B. Wang, F. Wang, Y. Wang, H. Chen, J. Yin and X. Chen, Sens. Actuators, B, 2015, 209, 1005–1010 CrossRef CAS.
- (a) M. Dong, Y.-W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed; (b) J. Y. Choi, G.-H. Kim, Z. Guo, H. Y. Lee, K. M. K. Swamy, J. Pai, S. Shin, I. Shin and J. Yoon, Biosens. Bioelectron., 2013, 49, 438–441 CrossRef PubMed.
- (a) X. Cao, W. Lin and Y. Ding, Chem. – Eur. J., 2011, 17, 9066–9069 CrossRef CAS PubMed; (b) H. Seo, M. E. Jun, K. Ranganathan, K.-H. Lee, K.-T. Kim, W. Lim, Y. M. Rhee and K. H. Ahn, Org. Lett., 2014, 16, 1374–1377 CrossRef CAS PubMed.
- (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC; (c) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (d) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285–2288 CrossRef CAS; (b) Y. Liu, F. Song, Z. Song, M. Liu and B. Yan, Org. Lett., 2005, 7, 5409–5412 CrossRef CAS PubMed; (c) X. Du, F. Song, Y. Lu, H. Chen and Y. Liu, Tetrahedron, 2009, 65, 1839–1845 CrossRef CAS; (d) C. H. Oh, H. J. Yi and K. H. Li, Bull. Korean Chem. Soc., 2010, 31, 683–688 CrossRef CAS.
- M. Üçüncü, E. Karakuş, M. Kuş, G. E. Akpınar, Ö. Aksın-Artok, N. Krause, S. Karaca, N. Elmacı and L. Artok, J. Org. Chem., 2011, 76, 5959–5971 CrossRef PubMed.
- See ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Absorbance and fluorescence data and all experimental procedures. See DOI: 10.1039/c6cc04100k |
| This journal is © The Royal Society of Chemistry 2016 |