Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Paramagnetic lanthanide chelates for multicontrast MRI†
Nevenka
Cakić
a,
Tanja
Savić
a,
Janice
Stricker-Shaver
a,
Vincent
Truffault
b,
Carlos
Platas-Iglesias
c,
Christian
Mirkes
d,
Rolf
Pohmann
d,
Klaus
Scheffler
*de and
Goran
Angelovski
*a
aMR Neuroimaging Agents, Max Planck Institute for Biological Cybernetics, 72076 Tübingen, Germany. E-mail: goran.angelovski@tuebingen.mpg.de
bMax Planck Institute for Developmental Biology, 72076 Tübingen, Germany
cCentro de Investigaciones Científicas Avanzadas (CICA) and Departamento de Química Fundamental, Universidade da Coruña, 15008 A Coruña, Spain
dHigh-Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, 72076 Tübingen, Germany. E-mail: klaus.scheffler@tuebingen.mpg.de
eDepartment for Biomedical Magnetic Resonance, University of Tübingen, 72076 Tübingen, Germany
First published on 2nd June 2016
Abstract
The preparation of a paramagnetic chelator that serves as a platform for multicontrast MRI, and can be utilized either as a T1-weighted, paraCEST or 19F MRI contrast agent is reported. Its europium(III) complex exhibits an extremely slow water exchange rate which is optimal for the use in CEST MRI. The potential of this platform was demonstrated through a series of MRI studies on tube phantoms and animals.
Visualization of various biological processes that take place at cellular and molecular levels is the main goal of modern diagnostic and molecular imaging techniques. Due to different sensitivity, penetration depth, spatial or temporal resolution properties of the available imaging methods, the development of multimodal imaging experienced great advancements in the last decade.1,2 Nevertheless, a combination of two (or more) techniques is often challenging, requiring integration of different physical phenomena in the common hardware, or careful design of multimodal imaging probes.3,4
Today, magnetic resonance imaging (MRI) is one of the most powerful imaging tools capable of displaying an excellent soft tissue contrast and furthermore different types of contrasts. A number of diverse MRI contrast agents can improve the specificity of MRI measurements and, based on their nature, can provide different types of information. The most commonly used 1H-MRI contrast agents are paramagnetic Gd3+-based complexes or superparamagnetic iron-oxide based nanoparticles (T1- and T2-shortening agents, respectively).5 Recently, an entirely different mechanism for altering an MRI contrast, based on chemical exchange saturation transfer (CEST), has been developed. Contrast agents for CEST imaging usually consist of paramagnetic chelates (paraCEST agents) specifically designed to shift the resonances of exchangeable protons (NH, OH or bound water) further away from the bulk water.6 One of the great advantages of CEST is the detectability of its effect in combination with the presaturation RF pulse; only when this RF is applied at the specific frequency of the exchangeable protons, the MRI contrast will appear. Consequently, this method allows the multi-frequency readout and adjustment of the frequencies (e.g. by choice of the group with exchangeable protons or choice of the paramagnetic ion), which can be used for separate detection and visualization of different cellular and tissue environments.7,8 Finally, MRI can be performed on heteronuclear and hyperpolarized systems.9 Here, the best choice is 19F NMR because of its high sensitivity, the easy re-tuning of standard MRI instruments from 1H to 19F nuclei, the high natural abundance of the 19F isotope and the absence of background signals from intrinsic biomolecules, thus allowing quantitative studies.10,11
With such potential to provide diverse information by employing different frequencies and contrast mechanisms, MRI could be exploited for concurrent studies by means of T1-weighted, CEST and 19F imaging protocols. To this aim, it is highly desirable to develop multicontrast agents, probes capable of making use of these different types of MR contrast, thus resulting in a set of unique information related to the studied tissue of interest.
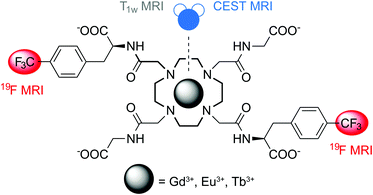
We therefore aimed to prepare an agent that is capable of chelating the paramagnetic ion, possesses frequency-shifted protons in slow exchange with bulk water, and finally bears a sufficient number of fluorine atoms to provide a sizeable 19F NMR signal (Fig. 1). We designed ligand L, a derivative of DOTA-tetraglycineamide (DOTAM-Gly),12 which displays CEST properties and ensures good solubility in aqueous solutions, especially after incorporation of fluorine atoms. The fluorinated moieties were introduced in the molecule at trans positions of the macrocyclic ring through two CF3 groups by using a 4-(trifluoromethyl)-L-phenylalanine (p-CF3-Phe) derivative and a convenient six-step synthetic procedure (ESI†). The resulting molecule L underwent complex formation with the Gd3+, Eu3+ and Tb3+ ions and the properties of GdL, EuL and TbL were further investigated (ESI†). Their overall charge should be advantageous (negative) due to the presence of four carboxylate groups, while appropriate complex stability necessary for in vivo experiments is ensured by the kinetic inertness provided by the tetraamide moieties.13 Finally, rigid benzyl moieties may establish, compared to e.g. aliphatic spacers, a well-defined distance of 19F nuclei from the paramagnetic center, thus providing advantageous relaxation properties at the frequency of these nuclei.14
The longitudinal relaxivity of GdL was calculated by obtaining T1 values of a series of buffered solutions at different complex concentrations. The resulting value of 3.1 mM−1 s−1 is expected for monohydrated tetraamide complexes with a slow water exchange rate.15 CEST measurements were performed by selective presaturation of the EuL and TbL samples in incremental steps over a range of frequencies followed by plotting the remaining steady-state bulk water signal, Mz/M0, as a function of saturation frequency (Fig. 2). The resulting Z-spectra of EuL exhibited a strong CEST effect (∼50% at 25 °C, 15 mM, B1 = 25.0 μT) centred at 50 ppm relative to bulk water, which is commonly associated with the water molecule directly coordinated to Eu3+. The CEST effect remains strong at 37 °C along with slight broadening and shifting of the CEST signal (5 ppm) towards the bulk water resonance due to the hyperfine shift effect of Eu3+ (Fig. 2, top).16
Water exchange rates at both temperatures were determined using a concentration-independent method previously developed by Sherry and colleagues,16 and were confirmed by quantification of the exchange rates as a function of saturation time or saturation power (QUEST and QUESP experiments, respectively).17 The values obtained from these experiments revealed extremely slow exchange rates of around 1 and 2 kHz or bound-water lifetimes (τM) of around 1 ms and 500 μs at 25 and 37 °C, respectively (Table 1). These residence times are as long as those reported very recently for the phosphonate esters of DOTAM-Gly at 25 °C,18 likely due to the presence of hydrophobic p-CF3-Phe moieties. Consequently, EuL displays an almost optimal τM which lies in the range of 10−4–10−2 s, allowing CEST experiments using weaker B1 fields. On the other hand, TbL displayed a weak CEST effect for both the inner-sphere water molecule and amide protons (ESI†), probably for the same reasons related to slow water exchange as in EuL.19,20
| Temperature | Omega plots | QUEST/QUESP |
|---|---|---|
| 25 °C | 1176 ± 22 | 930 ± 11 |
| 37 °C | 2036 ± 110 | 2000 ± 138 |
19F NMR spectra of GdL, EuL and TbL were recorded and 19F relaxation rates were determined to estimate the potential of these agents for 19F MRI. GdL displayed a single broad 19F NMR resonance, indicating substantial shortening of the relaxation times, while EuL and TbL revealed the existence of at least three paramagnetic species in the solution with different 19F chemical shifts (ESI†). While the two peaks with higher intensities can be assigned to the common monocapped square-antiprismatic and monocapped twisted square-antiprismatic (SAP and TSAP, respectively) isomers of this type of compound,21 the appearance of the third resonance with very weak intensity can be explained by racemization of the starting amino acid or racemization of alkylating arms prior to or during alkylation of cyclen, as previously observed for similar systems under comparable experimental conditions.22 Consequently, 19F relaxation rates were determined only for the most abundant peaks (Table 2). As expected, GdL dramatically affected both 19F R1 and R2, while still keeping a favorable R1/R2 ratio to obtain a good signal-to-noise ratio (SNR), when using ultrafast sequences.23EuL and TbL also enhanced 19F R1 and R2, however the reduction in the R1/R2 ratio (in the case of EuL) or larger signal splitting (in the case of TbL) suggests GdL as a better candidate for 19F MRI studies, with the current DOTA-type chelator.
The potential of these systems to serve as multicontrast agents was demonstrated using in vitro MRI on tube phantoms (Fig. 3a–d). Four tubes containing GdL, EuL, TbL and water as controls were imaged using a 7T MRI scanner by different imaging protocols and frequencies. The greatest effect on relaxation times and hence the signal enhancement in T1-weighted MRI experiments was observed for GdL (Fig. 3a), as would be expected; this effect was also confirmed by T2-weighted MRI experiments (Fig. 3b). All three complexes displayed very good 19F MRI contrasts using the sequence parameters adjusted to assume different 19F relaxation rates due to influence of different paramagnetic ions (Fig. 3c). Lastly, only EuL exhibited a strong contrast in the CEST MRI experiment (Fig. 3d), as already indicated above in the NMR CEST experiments.
To confirm this platform as a multicontrast MRI agent in a complex environment, we have carried out an MRI study on animals using GdL and EuL. The contrast agent was injected intracranially into the somatosensory cortex of the anesthetized rats outside the scanner. For GdL, the animal was transferred into the scanner, and a very strong T1-weighted MRI signal was recorded in vivo (Fig. 3e). Slow reduction in the MRI signal over a period of a few hours indicated very slow diffusion of GdL and its potential interaction with brain tissues. Although a similar behavior was previously observed with aminobisphosphonate-containing contrast agents,24 it is hard to rationalize this effect with the current chelating platform since its diffusion properties in solution did not indicate any aggregation, i.e. the diffusion coefficient corresponded to other monomeric agents of similar size (ESI†).
Additionally, the effect of EuL was assessed by means of MRI ex vivo. A weak T1-weigthed MRI contrast was obtained as expected after the animal was euthanized and transferred into the scanner (Fig. 3f). In parallel, a strong CEST contrast (∼10% signal change) was successfully obtained at the frequency of the inner-sphere water molecule bound to Eu3+ (Fig. 3g), confirming the great potential of EuL for further paraCEST studies due to its long τM (vide supra). In regard to 19F MRI, several imaging sequences were tested on both GdL and EuL, using non-fluorine containing anesthesia to avoid possible interferences at the fluorine frequency. However, only a slight change in signal intensity could be detected after 2.3 hours of monitoring. As already discussed above, the lack of signals could indicate an interaction of the agent with surrounding tissues, which significantly reduces 19F T2 relaxation times and leads to signal disappearance (a notable broadening of the 19F signal in the measured 1D spectrum was observed for GdL). However, this effect can likely be avoided with another experimental design where the tissue density is lower (e.g. in the blood stream or kidneys), or by combining this platform to a nanosized system that will prevent any interaction with the tissue and improve the agent's biocompatibility.25
In conclusion, we report a promising platform for the development of multicontrast agents for MRI. A small size molecule accommodates different paramagnetic ions and subsequently enhances 1H T1-weighted, 1H CEST, and 19F MRI contrasts. The GdL and EuL complexes can concurrently serve as 1H T1-weighted and 19F MRI, or as 1H CEST and 19F MRI agents, respectively. As these two complexes are expected to have essentially the same biodistribution, the three different contrast mechanisms could be exploited using the same molecular platform, even if GdL and EuL have to be administered separately.26 Furthermore, the installation of the aromatic fluorinated units likely caused extremely advantageous exchange rates in EuL for CEST experiments,20 while possibly deteriorating the agent's biocompatibility for 19F MRI. Nevertheless, further improvements should easily be envisaged to provide the optimized multicontrast agent. Structural optimizations towards adjustment of the chelator's coordination properties may lead to a single isomer species that will be beneficial for 19F MRI. Synthetic modifications can lead to a higher number of fluorine atoms per molecule to increase the signal intensity, while a combination with various nanocarriers can improve the delivery, biokinetics and potentiality also the T2 contrast of this multicontrast agent. The ability to collect different types of information from a single imaging probe just by using different imaging protocols brings new quality to MRI and can be a great asset for current molecular imaging to study various biological phenomena.
The authors thank Dr Kai Buckenmaier and Dr Dávid Balla for help in establishing appropriate anesthesia and MRI protocols and the Institute for Organic Chemistry, Faculty of Science, University of Tübingen, for the support in performing MS and elemental analyses. The financial support of the Max-Planck Society, the German Academic Exchange Service (DAAD, PhD fellowship to T.S.) and Ministerio de Economía y Competitividad (CTQ2013-43243-P and CTQ2015-71211-REDT, support to C. P.-I.) is gratefully acknowledged.
The animal experiments were approved by the local authorities (Regierungspraesidium), and were in compliance with the guidelines of the European directive (2010/63/EU) for the care and protection of animals used for scientific purposes.
Notes and references
- R. Weissleder and M. J. Pittet, Nature, 2008, 452, 580–589 CrossRef CAS PubMed.
- H. Kobayashi, M. R. Longmire, M. Ogawa and P. L. Choyke, Chem. Soc. Rev., 2011, 40, 4626–4648 RSC.
- S. R. Cherry, Annu. Rev. Biomed. Eng., 2006, 8, 35–62 CrossRef CAS PubMed.
- A. Y. Louie, Chem. Rev., 2010, 110, 3146–3195 CrossRef CAS PubMed.
- P. Caravan, in Molecular and cellular MR imaging, ed. M. M. J. Modo and J. W. M. Bulte, CRC Press, Taylor & Francis Group, Boca Raton, London, New York, 2007, pp. 13–36 Search PubMed.
- A. D. Sherry and M. Woods, Annu. Rev. Biomed. Eng., 2008, 10, 391–411 CrossRef CAS PubMed.
- S. Aime, C. Carrera, D. D. Castelli, S. G. Crich and E. Terreno, Angew. Chem., Int. Ed., 2005, 44, 1813–1815 CrossRef CAS PubMed.
- S. Viswanathan, S. J. Ratnakar, K. N. Green, Z. Kovacs, L. M. De Leon-Rodriguez and A. D. Sherry, Angew. Chem., Int. Ed., 2009, 48, 9330–9333 CrossRef CAS PubMed.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS PubMed.
- I. Tirotta, V. Dichiarante, C. Pigliacelli, G. Cavallo, G. Terraneo, F. B. Bombelli, P. Metrangolo and G. Resnati, Chem. Rev., 2015, 115, 1106–1129 CrossRef CAS PubMed.
- M. Srinivas, A. Heerschap, E. T. Ahrens, C. G. Figdor and I. J. M. d. Vries, Trends Biotechnol., 2010, 28, 363–370 CrossRef CAS PubMed.
- S. Aime, A. Barge, D. Delli Castelli, F. Fedeli, A. Mortillaro, F. U. Nielsen and E. Terreno, Magn. Reson. Med., 2002, 47, 639–648 CrossRef CAS PubMed.
- E. Brücher, G. Tircsó, Z. Baranyai, Z. Kovács and A. D. Sherry, The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, John Wiley & Sons, Ltd, 2013, pp. 157–208 Search PubMed.
- P. Harvey, I. Kuprov and D. Parker, Eur. J. Inorg. Chem., 2012, 2015–2022 CrossRef CAS.
- S. Aime, A. Barge, J. I. Bruce, M. Botta, J. A. K. Howard, J. M. Moloney, D. Parker, A. S. de Sousa and M. Woods, J. Am. Chem. Soc., 1999, 121, 5762–5771 CrossRef CAS.
- W. T. Dixon, J. M. Ren, A. J. M. Lubag, J. Ratnakar, E. Vinogradov, I. Hancu, R. E. Lenkinski and A. D. Sherry, Magn. Reson. Med., 2010, 63, 625–632 CrossRef CAS PubMed.
- M. T. McMahon, A. A. Gilad, J. Y. Zhou, P. Z. Sun, J. W. M. Bulte and P. C. M. van Zijl, Magn. Reson. Med., 2006, 55, 836–847 CrossRef CAS PubMed.
- W. S. Fernando, A. F. Martins, P. Zhao, Y. Wu, G. E. Kiefer, C. Platas-Iglesias and A. D. Sherry, Inorg. Chem., 2016, 55, 3007–3014 CrossRef CAS PubMed.
- X. J. Wang, Y. K. Wu, T. C. Soesbe, J. Yu, P. Y. Zhao, G. E. Kiefer and A. D. Sherry, Angew. Chem., Int. Ed., 2015, 54, 8662–8664 CrossRef CAS PubMed.
- A. D. Sherry and Y. K. Wu, Curr. Opin. Chem. Biol., 2013, 17, 167–174 CrossRef CAS PubMed.
- J. A. Peters, K. Djanashvili, C. F. G. C. Geraldes and C. Platas-iglesias, The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, John Wiley & Sons, Ltd, 2013, pp. 209–276 Search PubMed.
- T. Mani, A. C. L. Opina, P. Y. Zhao, O. M. Evbuomwan, N. Milburn, G. Tircso, C. Kumas and A. D. Sherry, JBIC, J. Biol. Inorg. Chem., 2014, 19, 161–171 CrossRef CAS PubMed.
- F. Schmid, C. Höltke, D. Parker and C. Faber, Magn. Reson. Med., 2013, 69, 1056–1062 CrossRef CAS PubMed.
- I. Mamedov, S. Canals, J. Henig, M. Beyerlein, Y. Murayama, H. A. Mayer, N. K. Logothetis and G. Angelovski, ACS Chem. Neurosci., 2010, 1, 819–828 CrossRef CAS PubMed.
- K. J. Thurecht, I. Blakey, H. Peng, O. Squires, S. Hsu, C. Alexander and A. K. Whittaker, J. Am. Chem. Soc., 2010, 132, 5336–5337 CrossRef CAS PubMed.
- A. M. Funk, V. Clavijo Jordan, A. D. Sherry, S. J. Ratnakar and Z. Kovacs, Angew. Chem., Int. Ed., 2016, 55, 5024–5027 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, descriptions of CEST, 19F NMR, NMR diffusion and MRI experiments. See DOI: 10.1039/c6cc04011j |
| This journal is © The Royal Society of Chemistry 2016 |