 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A modular approach towards functional supramolecular aggregates – subtle structural differences inducing liquid crystallinity†
Michael
Pfletscher
a,
Christoph
Wölper
b,
Jochen S.
Gutmann
c,
Markus
Mezger
d and
Michael
Giese
*a
aInstitut für Organische Chemie, Universität Duisburg-Essen, Universitätsstr. 7, 45141 Essen, Germany. E-mail: michael.giese@uni-due.de; Web: http://www.gieselab.de
bInstitut für Anorganische Chemie, Universität Duisburg-Essen, Universitätsstr. 7, 45141 Essen, Germany
cInstitut für Physikalische Chemie und CENIDE, Universität Duisburg-Essen, Universitätsstr. 7, 45141 Essen, Germany
dInstitut für Physik, Johannes Gutenberg-Universität Mainz und Max-Planck-Institut für Polymerforschung, Ackermannweg 10, 55021 Mainz, Germany
First published on 17th June 2016
Abstract
Herein we report an efficient modular approach to supramolecular functional materials. Hierarchical self-assembly of azopyridine derivatives and hydrogen-bond donors yielded discotic assemblies. Subtle differences in the core units introduced mesomorphic behaviour and fast photo-response of the liquid crystals based on phloroglucinol. The presented results prove the benefits of a modular methodology towards highly responsive materials with tailor-made properties.
Within the past two decades supramolecular chemistry has developed into a powerful tool to create novel functional materials with fascinating properties.1 The employment of non-covalent forces provides many advantages compared to conventional methods and facilitates fabrication, processing and recycling.2 Simple mixing of pre-tailored building blocks at room temperature yields highly functional aggregates via self-assembly and allows dynamic response on external stimuli or damages (self-healing/-repair).1a,3
Supramolecular liquid crystals are functional assemblies showing structural complexity as well as molecular order.4 The use of hydrogen-bonds to design novel liquid crystals is appealing and pioneering works of Kato, Fréchet5 and Lehn6 already yielded supramolecular assemblies with mesomorphic properties.7 However, since the self-assembly process depends on a complex interplay of a variety of non-covalent forces the rational design and prediction of properties remains challenging.1d,4a,8
Azobenzenes are frequently used as photo-responsive switches showing reversible trans/cis isomerization upon photoirradiation.9 Azopyridine (Ap) derivatives are promising candidates to develop responsive supramolecular materials since they combine the photo-active azobenzene group with the hydrogen/halogen bond-acceptor capability of the pyridyl-group.5,10
As already mentioned above a number of hydrogen-bonded liquid crystals were already described. However, the rational design of novel functional materials based on supramolecular assemblies with predictable properties requires a fundamental understanding of the underlying principles and efficient methods to screen a broad variety of influencing factors. Therefore, we herein introduce a modular approach to tailor-made supramolecular liquid crystals (smLCs) by using the principles of crystal engineering and self-assembly. Our first attempt combines commercially available core units (trimesic acid (TA), cyanuric acid (CA) and phloroglucinol (PG)) and simple non-mesogenic azopyridines led to a series of C3-symmetric assemblies with liquid crystalline behaviour as well as fast and reversible response to irradiation with light. The intrinsic flexibility of the chosen methodology allows an efficient screening of a wide variety of molecules as potential building blocks for functional materials and provides the opportunity for systematic studies on the structure–property relationship of liquid crystalline assemblies. These fundamental studies are crucial towards the design of efficient functional materials.4d,11 Inspired by well-established supramolecular synthons12 in crystal engineering and supramolecular chemistry promising candidates were selected to create hydrogen-bonded assemblies with liquid crystalline properties. Our initial attempt combines a series of hydrogen-bond donor core units with seven different hydrogen-bond acceptors based on azopyridine (Ap-3–10, blue, see Scheme 1). Mixing the core units with the azopyridines in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 leads to discotic assemblies, which were investigated in respect to their thermal properties and mesomorphic behavior. So far just a few other examples are reported employing hydrogen bonds to create mesogenic assemblies.8,13 In most of these examples at least one of the building block showed mesomorphic behaviour before complexation and no photo-switchable system was described. However, we herein focussing on the modular design of approach allowing for the efficient screening of building blocks and the unique combination their individual properties in a supramolecular assembly.
3 leads to discotic assemblies, which were investigated in respect to their thermal properties and mesomorphic behavior. So far just a few other examples are reported employing hydrogen bonds to create mesogenic assemblies.8,13 In most of these examples at least one of the building block showed mesomorphic behaviour before complexation and no photo-switchable system was described. However, we herein focussing on the modular design of approach allowing for the efficient screening of building blocks and the unique combination their individual properties in a supramolecular assembly.
In order to screen the modular tool box for promising combinations, the commercially available core units were mixed with the azopyridine derivatives in acetone. After removing the solvent the thermal properties of the aggregates were studied under the polarized optical microscope (POM) in a temperature range of 25–150 °C. It should be noticed that none of the individual starting materials exhibited mesogenic character. Although all three selected core units exhibit C3-symmetry and the general ability to form aggregates with the azopyridine moieties, tremendous differences were found in the mesomorphic behaviour of the assemblies. Intuitively, strong intermolecular interactions are expected between the azopyridine unit and the trimesic cyanuric acid assemblies, while significantly weaker interactions are expected for the hydrogen-bonded phloroglucinol aggregates. However, to our surprise exclusively the phloroglucinol exhibited the formation of stable complexes as proven by POM. The POM images of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixtures of trimesic acid and cyanuric acid with Ap-6, 8 and 10 revealed inhomogeneous melting (Fig. 1A, Fig. S1 and S2, ESI†). In contrast, the phloroglucinol assemblies melted to a homogeneous isotropic phase (Fig. 1B and Fig. S3, ESI†).
3 mixtures of trimesic acid and cyanuric acid with Ap-6, 8 and 10 revealed inhomogeneous melting (Fig. 1A, Fig. S1 and S2, ESI†). In contrast, the phloroglucinol assemblies melted to a homogeneous isotropic phase (Fig. 1B and Fig. S3, ESI†).
This observation is attributed to the disaggregation of the hydrogen-bonded trimesic and cyanuric acid aggregates with azopyridine in favour of the stronger hydrogen bonding within the phase separated homo-crystals of the cyanuric and trimesic acid. This is in-line with the significantly higher melting points of trimesic acid (380 °C) and cyanuric acid (320 °C) in respect to phloroglucinol (215 °C). Upon cooling from the isotropic liquid the phloroglucinol series showed a Schlieren texture, characteristic for a nematic mesophase. Therefore, in the following discussion we will focus on the aggregation and characterization of the phloroglucinol system. The hydrogen-bonded aggregates PG⋯(Ap-3)3–PG⋯(Ap-10)3 were obtained by mixing solutions of PG and the corresponding Ap in acetone or by heating mixtures of the pristine starting compounds in a microwave oven. Complexation was confirmed by IR-spectroscopy, single crystal analysis and polarized optical microscopy. Comparison of the IR-spectra (Fig. S4, ESI†) of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 PG
3 PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ap complexes clearly demonstrates a shift of the broad OH-signal of the phloroglucinol from ∼3200 cm−1 to ∼3050 cm−1 and the appearance of a signal at ∼2630 cm−1 indicating the formation of the hydrogen-bonded complex. Similar results were found for the whole series of investigated liquid crystals (Fig. S5, ESI†). POM images of PG⋯(Ap-6)1 and PG⋯(Ap-6)2 show the presence of PG crystallites even at 150 °C due to incomplete formation of the hydrogen bonded assembly (Fig. S3, ESI†). Homogeneous melting as well as stable liquid crystalline phases were exclusively found for the 1
Ap complexes clearly demonstrates a shift of the broad OH-signal of the phloroglucinol from ∼3200 cm−1 to ∼3050 cm−1 and the appearance of a signal at ∼2630 cm−1 indicating the formation of the hydrogen-bonded complex. Similar results were found for the whole series of investigated liquid crystals (Fig. S5, ESI†). POM images of PG⋯(Ap-6)1 and PG⋯(Ap-6)2 show the presence of PG crystallites even at 150 °C due to incomplete formation of the hydrogen bonded assembly (Fig. S3, ESI†). Homogeneous melting as well as stable liquid crystalline phases were exclusively found for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometries (Fig. S6, ESI†).
3 stoichiometries (Fig. S6, ESI†).
In order to gain further insight in the molecular structure of the complexes, single crystals of PG⋯(Ap-4)3 were obtained by slow evaporation of a solutions of PG and Ap-4 in acetone. PG⋯(Ap-4)3 crystallizes in the centro-symmetric space group P21/c with two hydrogen-bonded assemblies per asymmetric unit cell (see Fig. 2).
The crystal structure clearly shows the hydrogen-bridges between the PG core and the Ap-4 units (Npyr⋯HOPG = 1.82–1.96 Å) and reveals some disorder of the azocompound. The planes of the azopyridine moieties are oriented out of plane in respect to the phloroglucinol (54.9°, 63.6° and 86.1°) unit to form V-shaped assemblies, which are stacked into each other related by 21 symmetry. The phloroglucinol moieties of adjacent hydrogen-bonded aggregates are oriented in the same plane and show non-classical hydrogen-bonding (CHPG⋯OHPG = 2.37 Å). Interestingly, attempts to crystallize the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 assemblies yielded individual crystals of PG⋯(Ap-4)3 and PG. The crystal structure combined with the obtained IR-spectra and the POM images clarifies the relevance of the hydrogen-bonding and 1
2 assemblies yielded individual crystals of PG⋯(Ap-4)3 and PG. The crystal structure combined with the obtained IR-spectra and the POM images clarifies the relevance of the hydrogen-bonding and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometry of the complexes in order to observe stable mesophases.
3 stoichiometry of the complexes in order to observe stable mesophases.
The thermal behavior of the mesogens and their individual components were characterized by POM, differential scanning calorimetry (DSC) and X-ray scattering (XRD). As already mentioned above the individual components of the hydrogen-bonded assembly (PG and Ap-3–Ap-10) did not show mesomorphic behavior as proven by POM. The same was found for PG⋯(Ap-3)3, which is not a liquid crystal and melts isotropically at 142 °C. However, the hydrogen-bonded assemblies of PG with Ap-4–Ap-10 clearly show the characteristic textures of a nematic mesophase upon cooling the samples from ∼110 °C to room temperature (Fig. 3 and Fig. S7, ESI†). In order to quantify the observations made by POM, DSC profiles of the hydrogen-bonded assemblies were collected (Fig. S7 and Table S8, ESI†). Comparison of the thermal properties indicates a significant stabilization of the mesophase upon elongation of the alkylchain at the peripheral unit. Changes of ΔT = 5.6 °C for PG⋯(Ap-4)3 to ΔT = 26.7 °C for PG⋯(Ap-9)3 were observed. Interestingly, exclusively the PG⋯(Ap-8)3 and PG⋯(Ap-9)3 aggregates revealed enantiotropic phase transition showing mesogenic behavior upon cooling and heating. However, the mesophases upon heating are found to be quite narrow (∼4 °C, Fig. S7E and F, ESI†). In contrast, a related system reported by Jho and coworkers13j exhibited an enantiotropic mesophase range of more than 40 °C. This difference is most likely attributed to the repulsive forces of the azo-groups and again shows how subtle structural changes significantly influence the properties of supramolecular materials.
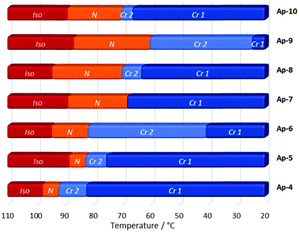 | ||
| Fig. 3 Monotropic phase transition temperatures upon cooling (10 K min−1) of the PG⋯(Ap-N)3 assemblies as observed by DSC (Iso = isotropic phase, N = nematic phase, Cr = crystalline state). | ||
XRD measurements on PG⋯(Ap-6)3 confirmed the formation of nematic mesophases (Fig. 4). In the isotropic melt (100 °C), the diffraction patterns show two diffuse halos at 2θ = 3.5° and 20°. This corresponds to real space distances of 25 Å and 4.5 Å respectively. Upon cooling to 90 °C in a vertical magnetic field of approx. 500 G two sets of diffuse peaks at 3.2° and 20° are observed. They are oriented perpendicular to each other, confirming the formation of a nematic phase.
 | ||
| Fig. 4 X-ray diffraction pattern of PG⋯(Ap-6)3 in the isotropic (left) and nematic phase (right) in a magnetic field. | ||
The peripheral units of the hydrogen-bonded complexes are based on azopyridine, enabling fully reversible trans/cis isomerization when irradiated with UV-light.9a,14 The UV-Vis-spectra of thin films of the molten liquid crystals were obtained showing strong absorption in the region of 280 to 410 nm with an absorption maximum at 365 nm (Fig. S8, ESI†).
In order to investigate the photo-responsive properties of the liquid crystals, the samples were irradiated with a laser pointer (405 nm, 5 mW) in their mesophases. The effect was in situ followed by POM. For all investigated assemblies the same effect was found. Initially the liquid crystal is present in its trans-state showing the characteristic Schlieren texture of a nematic phase. However, upon irradiation at 405 nm the birefringent liquid crystal texture disappeared instantaneously (see Fig. 5 and Fig. S9, Video S1, ESI†) demonstrating a photo-induced phase transition from the meso- to the isotropic phase. The phase transition is attributed to the photo-isomerization of the azopyridine units from their trans to cis form as the bent-shaped cis-isomers tend to destabilized the order of the mesophase.15 As soon as the irradiation is stopped the mesophase is recovered within seconds, which is in accordance to previous results found for the thermal relaxation of azopyridines.9a,14
In order to confirm that the observed photoinduced phase transition is caused by cis/trans isomerization of the azopyridine, the same sample was irradiated with a green (532 nm, 5 W) and a red laser pointer (650 nm, 5 mW). No effect on the mesophases was observed (Fig. S10 and S11, Videos S2 and S3, ESI†). Therefore, we attribute the previously observed phase transition to the isomerization of the azopyridine moiety and not by local thermal excitation.
In summary, we reported an approach to supramolecular functional assemblies based on azopyridine derivatives. A series of novel hydrogen-bonded liquid crystals were synthesized and characterized exhibiting rapid photo-response, which makes these materials appealing for applications in organic opto-electronics such as sensors or optical gates.16 The present study is a proof of principle and demonstrates the utility of a modular design allowing efficient screening of a plethora of supramolecular building blocks towards the design of novel functional materials and fundamental studies on the structure–property relationship of liquid crystalline aggregates. Our current research is now evaluating the scope of this approach in respect to establish columnar phases, which have great potential in respect to electronic and photonic devices.4d,17
M. G. and M. P. were generously supported by the Professor Werdelmann Stiftung and the Fonds der chemischen Industrie. We thank Professor Christian Mayer and Kirsten Schwark for their experimental assistance (DSC).
References
-
(a) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC
; (b) K. Liu, Y. Kang, Z. Wang and X. Zhang, Adv. Mater., 2013, 25, 5530 CrossRef CAS PubMed
; (c) R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498 CrossRef CAS PubMed
; (d) D. González-Rodríguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310 CrossRef
.
- Y. Long, J.-f. Hui, P.-p. Wang, G.-l. Xiang, B. Xu, S. Hu, W.-C. Zhu, X.-Q. Lü, J. Zhuang and X. Wang, Sci. Rep., 2012, 2, 612 CrossRef PubMed
.
-
(a) Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446 RSC
; (b) G. Li, J. Wu, B. Wang, S. Yan, K. Zhang, J. Ding and J. Yin, Biomacromolecules, 2015, 16, 3508 CrossRef CAS PubMed
; (c) Y.-G. Jia and X. X. Zhu, Chem. Mater., 2015, 27, 387 CrossRef CAS
; (d) M. Nakahata, Y. Takashima and A. Harada, Macromol. Rapid Commun., 2016, 37, 86 CrossRef CAS PubMed
.
-
(a) T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS PubMed
; (b) T. Kato, T. Yasuda, Y. Kamikawa and M. Yoshio, Chem. Commun., 2009, 729 RSC
; (c) C. Tschierske, Angew. Chem., Int. Ed., 2013, 52, 8828 CrossRef CAS PubMed
; (d) T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139 CrossRef PubMed
.
-
(a) T. Kato and J. M. J. Fréchet, J. Am. Chem. Soc., 1989, 111, 8533 CrossRef CAS
; (b) T. Kato, T. Uryu, F. Kaneuchi, C. Jin and J. M. J. Fréchet, Liq. Cryst., 1993, 14, 1311 CrossRef CAS
; (c) T. Kato, T. Kato, J. M. J. Fréchet, T. Uryu, F. Kaneuchi, C. Jin and J. M. J. Fréchet, Liq. Cryst., 2006, 33, 1429 CrossRef CAS
.
-
(a) M. Suárez, J.-M. Lehn, S. C. Zimmerman, A. Skoulios and B. Heinrich, J. Am. Chem. Soc., 1998, 120, 9526 CrossRef
; (b) M.-J. Brienne, J. Gabard, J.-M. Lehn and I. Stibor, J. Chem. Soc., Chem. Commun., 1989, 1868 RSC
.
-
(a) C. Domínguez, B. Heinrich, B. Donnio, S. Coco and P. Espinet, Chem. – Eur. J., 2013, 19, 5988 CrossRef PubMed
; (b) C. Dominguez, B. Donnio, S. Coco and P. Espinet, Dalton Trans., 2013, 42, 15774 RSC
; (c) A. B. Miguel-Coello, M. Bardají, S. Coco, B. Donnio, B. Heinrich and P. Espinet, Inorg. Chem., 2014, 53, 10893 CrossRef CAS PubMed
.
- M. Lehmann, Chem. – Eur. J., 2009, 15, 3638 CrossRef CAS PubMed
.
-
(a) J. García-Amorós and D. Velasco, Beilstein
J. Org. Chem., 2012, 8, 1003 CrossRef PubMed
; (b) A. Goulet-Hanssens and C. J. Barrett, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3058 CrossRef CAS
; (c) D. Iqbal and M. Samiullah, Materials, 2013, 6, 116 CrossRef CAS PubMed
; (d) C. Zong, Y. Zhao, H. Ji, X. Han, J. Xie, J. Wang, Y. Cao, S. Jiang and C. Lu, Angew. Chem., Int. Ed., 2016, 55, 3931 CrossRef CAS PubMed
; (e) Y. Norikane, Y. Hirai and M. Yoshida, Chem. Commun., 2011, 47, 1770 RSC
; (f) S. Yagai, T. Nakajima, T. Karatsu, K.-I. Saitow and A. Kitamura, J. Am. Chem. Soc., 2004, 126, 11500 CrossRef CAS PubMed
; (g) S. Yagai, T. Nakajima, K. Kishikawa, S. Kohmoto, T. Karatsu and A. Kitamura, J. Am. Chem. Soc., 2005, 127, 11134 CrossRef CAS PubMed
.
-
(a) Y. Chen, H. Yu, L. Zhang, H. Yang and Y. Lu, Chem. Commun., 2014, 50, 9647 RSC
; (b) O. S. Bushuyev, T. C. Corkery, C. J. Barrett and T. Friscic, Chem. Sci., 2014, 5, 3158 RSC
.
-
(a) R. J. Bushby and O. R. Lozman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 569 CrossRef CAS
; (b) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC
.
-
(a) G. R. Desiraju, Angew. Chem., Int. Ed., 1995, 34, 2311 CrossRef CAS
; (b) A. Mukherjee, Cryst. Growth Des., 2015, 15, 3076 CrossRef CAS
.
-
(a) A. A. Vieira, H. Gallardo, J. Barbera, P. Romero, J. L. Serrano and T. Sierra, J. Mater. Chem., 2011, 21, 5916 RSC
; (b) A. A. Vieira, E. Cavero, P. Romero, H. Gallardo, J. L. Serrano and T. Sierra, J. Mater. Chem. C, 2014, 2, 7029 RSC
; (c) A. Kohlmeier and D. Janietz, Liq. Cryst., 2007, 34, 289 CrossRef CAS
; (d) S. J. Lee, M. K. You, S. W. Lee, J. Lee, J. H. Lee and J. Y. Jho, Liq. Cryst., 2011, 38, 1289 CrossRef CAS
; (e) J. H. Lee, I. Jang, S. H. Hwang, S. J. Lee, S. H. Yoo and J. Y. Jho, Liq. Cryst., 2012, 39, 973 CrossRef CAS
; (f) B. Feringán, P. Romero, J. L. Serrano, R. Giménez and T. Sierra, Chem. – Eur. J., 2015, 21, 8859 CrossRef PubMed
; (g) S. Yagai, Y. Goto, T. Karatsu, A. Kitamura and Y. Kikkawa, Chem. – Eur. J., 2011, 17, 13657 CrossRef CAS PubMed
; (h) M.-H. Ryu, J.-W. Choi, H.-J. Kim, N. Park and B.-K. Cho, Angew. Chem., Int. Ed., 2011, 50, 5737 CrossRef CAS PubMed
; (i) H. Blanco, V. Iguarbe, J. Barberá, J. L. Serrano, A. Elduque and R. Giménez, Chem. – Eur. J., 2016, 22, 4924 CrossRef CAS PubMed
; (j) H. Lee, M.-J. Han, S. H. Hwang, I. Jang, S. J. Lee, S. H. Yoo, J. Y. Jho and S.-Y. Park, Tetrahedron Lett., 2005, 46, 7143 CrossRef
; (k) H.-K. Lee, H. Lee, Y. H. Ko, Y. J. Chang, N.-K. Oh, W.-C. Zin and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2669 CrossRef CAS
.
- J. Garcia-Amoros, S. Nonell and D. Velasco, Chem. Commun., 2011, 47, 4022 RSC
.
-
(a) H. Yu, H. Liu and T. Kobayashi, ACS Appl. Mater. Interfaces, 2011, 3, 1333 CrossRef CAS PubMed
; (b) H. Liu, T. Kobayashi and H. Yu, Macromol. Rapid Commun., 2011, 32, 378 CrossRef CAS PubMed
.
-
(a) H. Yu, J. Mater. Chem. C, 2014, 2, 3047 RSC
; (b) V. Chigrinov, A. Muravski, H. S. Kwok, H. Takada, H. Akiyama and H. Takatsu, Phys. Rev. E, 2003, 68, 061702 CrossRef PubMed
; (c) O. Yaroshchuk and Y. Reznikov, J. Mater. Chem., 2012, 22, 286 RSC
; (d) T. Seki, S. Nagano and M. Hara, Polymer, 2013, 54, 6053 CrossRef CAS
; (e) M. Giese, T. Krappitz, R. Y. Dong, C. A. Michal, W. Y. Hamad, B. O. Patrick and M. J. MacLachlan, J. Mater. Chem. C, 2015, 3, 1537 RSC
.
-
(a) Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS PubMed
; (b) S. Yazaki, M. Funahashi, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2010, 132, 7702 CrossRef CAS PubMed
; (c) T. Yasuda, T. Shimizu, F. Liu, G. Ungar and T. Kato, J. Am. Chem. Soc., 2011, 133, 13437 CrossRef CAS PubMed
; (d) B. Soberats, M. Yoshio, T. Ichikawa, X. Zeng, H. Ohno, G. Ungar and T. Kato, J. Am. Chem. Soc., 2015, 137, 13212 CrossRef CAS PubMed
; (e) H. N. Tsao, H. J. Räder, W. Pisula, A. Rouhanipour and K. Müllen, Phys. Status Solidi A, 2008, 205, 421 CrossRef CAS
; (f) V. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H. W. Spiess, S. D. Hudson and H. Duan, Nature, 2002, 417, 384 CrossRef PubMed
; (g) W. Pisula, M. Zorn, J. Y. Chang, K. Müllen and R. Zentel, Macromol. Rapid Commun., 2009, 30, 1179 CrossRef CAS PubMed
; (h) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC
; (i) S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hägele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Angew. Chem., Int. Ed., 2007, 46, 4832 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1455700. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc03966a |
| This journal is © The Royal Society of Chemistry 2016 |




