Open Access Article
Open Access ArticleElectron–phonon coupling in engineered magnetic molecules†
Violeta
Iancu
*ab,
Koen
Schouteden
a,
Zhe
Li
a and
Chris
Van Haesendonck
a
aLaboratory of Solid-State Physics and Magnetism, KU Leuven, BE-3001 Leuven, Belgium. E-mail: Violeta.Iancu@eli-np.ro; Chris.VanHaesendonck@fys.kuleuven.be
bExtreme Light Infrastructure – Nuclear Physics/Horia Hulubei National Institute for R&D in Physics and Nuclear Engineering, Bucharest-Magurele, RO-077125, Romania
First published on 25th August 2016
Abstract
We probe the electron–phonon coupling for in situ engineered porphyrin-based magnetic molecular layers supported on weakly reactive surfaces. Using high-resolution scanning tunneling microscopy and spectroscopy at 4.5 K we show that the electronic and magnetic properties of the engineered molecules are the result of interplay between many-body spin–flip excitations and electron–phonon interactions.
Metallo-porphyrins are widely found in biological systems (chlorophyll-a, heme) and their functionality is further explored for use in artificial systems.1 Metallo-porphyrins are easily vapor-deposited and adsorbed on metallic surfaces to mimic and investigate possible working configurations in future molecular devices.2 Tetrapyridyl pophyrin (5,10,15,20-tetra(4-pyridyl)-21H, 23H-porphine, TPyP), a porphyrine derivate investigated in this work, has shown great versatility in terms of self-assembly, doping and property tuning.3–6 For instance on an atomically clean Au(111) surface, TPyP can form a large variety of molecular structures with different packing densities,5,7,8 whereas on Ag(111) the molecules form only close-packed structures.3,4,6 The versatility of these molecules extends beyond their 2D assembly capabilities and by a simple magnetic atom doping their properties can be conveniently tailored ad hoc.6,9 Numerous experimental and theoretical studies showed how electrons (from leads and supporting substrates) interacting with molecules can have exciting consequences and reveal interesting phenomena among which Kondo effect,10–14 negative-differential resistance,15,16 Coulomb blockade,17 and vibronic coupling.18
Here, we investigate the electronic transport through magnetic TPyP molecules supported by a Au(111) surface and measured with a tungsten scanning tunneling microscope (STM) tip to probe electron–phonon coupling. The magnetic molecules were synthesized in situ through chemical reactions between non-metallic porphyrin-based molecules and magnetic (Co, Cr) atoms, an established procedure to create and study exotic molecules in a highly controlled environment.6,9,19–21 Conductance measurements through molecules at low temperatures reveal pronounced Kondo resonances at zero bias and additional Kondo resonance replicas observed at higher voltages in vibrating CoTPyP molecules. The Kondo resonance replicas are observed only in the presence of electron–phonon interactions and are associated with creation and destruction of phonons.22 Adatom coverages in excess of one adatom per molecule lead to the formation of highly organized structures of magnetic molecules and metallic clusters.
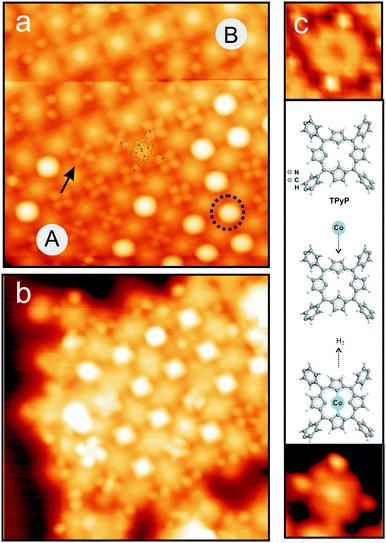
Fig. 1 presents the TPyP molecules adsorbed on Au(111) after doping with Co (Fig. 1(a)) and Cr (Fig. 1(b)) atoms.23 The TPyP molecules are non-metallic porphyrin-based systems that consist of a porphyrin macrocycle and four pyridyl groups at the periphery. By doping we mean the substitutional insertion of a single magnetic atom at the center of the porphyrin macrocycle, as is schematically described in Fig. 1(c).24 The TPyP molecules were vapor-deposited (at 550 K) and adsorbed on a clean Au(111) surface held at room temperature to form close-packed molecular islands (see Section I in ESI† for more details on TPyP self-assembly on Au(111)). Minute amount of either Co or Cr (0.09 monolayer (ML) of Co and 0.05 ML of Cr; 1 ML = 1.38 × 1015 atoms per cm2) were subsequently deposited onto the close-packed TPyP structures kept at room temperature. Further annealing to 530 K for 60 minutes was used to activate the chemical reactions and to achieve homogeneously doped molecular layers. The successful molecular doping is inferred from visual inspection of the STM images and electronic property measurements of the molecules (discussed later in the article). The appearance of the molecule changes upon doping: the original depression at the center transforms into a protrusion after atom insertion (Fig. 1(c)). Measurements of the molecular apparent height indicate about 0.6 Å increase upon atom insertion, an effect that was also observed for porphyrins doped with Fe, Co or Cr atoms.6,9
The effect that magnetic atom deposition has on the self-assembled TPyP molecules depends on their packing density. For instance, the Co/Cr atomic depositions onto the close-packed monolayer structures preserve the self-organization of the molecules, whereas the TPyP porous monolayer structures are destroyed by addition of Co/Cr atoms (see Fig. S2 from ESI† for more details). The close-packed structures that prevail are mostly square-lattice arrangements like those shown for CoTPyP and for CrTPyP in Fig. 1(a) and (b) but not always (see pattern B in Fig. 1(a)). Pattern A displays a square lattice with nearest neighbour separation of 1.4 nm; in this case the pyridyl groups are engaged in four-fold bonds (see Fig. S1 in ESI† for a schematic illustration of the network structures). The second pattern, pattern B, consists of alternating rows of molecules, with molecules in two adjacent rows having different orientation. Pattern B has an oblique unit cell formed by a mixture of 2- and 3-fold interacting pyridyls as seen in the schematic drawing presented in Fig. S1 of ESI.†
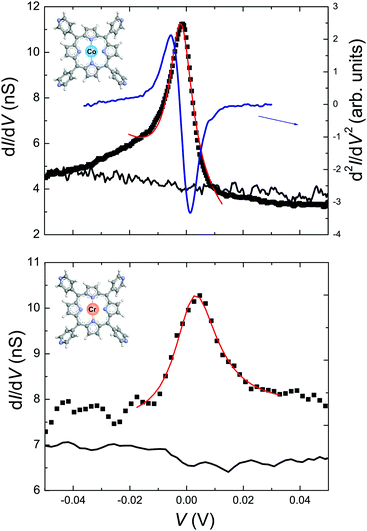
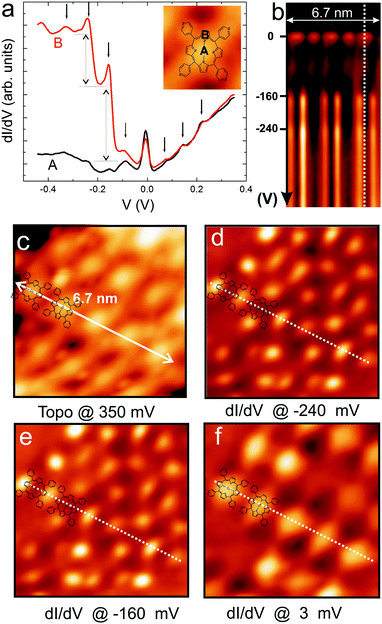
Measurements of the local density of states (LDOS) around the Fermi level performed at 4.5 K reveal the magnetic properties of CoTPyP and CrTPyP molecules and confirm the metalation of the porphyrin macrocycle by the magnetic centers. Fig. 2 displays high-resolution dI/dV spectra measured on the CoTPyP, CrTPyP, and on the bare substrate as well as the d2I/dV2 spectrum measured at the center of CoTPyP. A very pronounced zero bias peak (ZBP) is detected at the center of both molecules and is interpreted as a Kondo resonance.25,26 The asymmetric shape of the CoTPyP ZBP may be due to a near filled state peak, which was also observed in other Kondo systems adsorbed on Au(111).14,27 The observed Kondo resonances reflect the screened magnetic moment and advocate the magnetic nature of the molecules.6 The corresponding Kondo temperature was determined by Fano-line fitting28,29 of the ZBP. At 4.5 K, TK equals 53 ± 10 K for CoTPyP and 106 ± 15 K for CrTPyP.30 Interestingly, the dI/dV spectra of the CoTPyP molecules display additional peaks on both sides of the ZBP as illustrated in Fig. 3(a). These side peaks repeat every 80 mV and are accompanied by two step-like increases at biases of −160 and −240 mV, respectively. The step-like increases in dI/dV spectra are well-known signatures of molecular vibrations.31 We assign the side peaks observed in CoTPyP to Kondo resonance replicas based on previous studies that show that electronic transport through vibrating molecules can lead to emergence of peaks in the conductance18,32 or to the appearance of Kondo replica at energies (biases) that match multiples of phonons frequencies (see Fig. S3 in ESI† for an illustration of the tunneling processes involved and and see Fig. S4 for dI/dV spectra in a broader voltage range revealing more replicas).22,33 Kondo resonance replicas, also referred to as vibrational Kondo resonances, have been observed for organic charge–transfer complexes34 at single phonon energy and have not yet been observed for porphyrin-based molecules. In CoTPyP, the ZBP and the 80 mV-spaced peaks are observed on the entire porphyrin macrocycle (see Fig. 3(a)). The spatial extent of the magnetic and vibrational features of the molecules are best visualized in dI/dV maps (see Fig. 3(b) and (d)–(f)), which help explain their origin. The ZBP signal is dominant across the whole porphyrin macrocycle, which suggests hybridization between the metal center and the ligand (Fig. 3(f)). The −160 and −240 mV vibrational features emerge at the periphery of the macrocycle, more exactly near two opposite pyrrole groups (see Fig. 3(d) and (e)). By comparison with available experimental data and theoretical calculation of vibrational modes in similar molecules8,35–37 (see Table S1 of ESI†) we may assign the 160 mV molecular vibrations to stretching of C–N and C–C bonds around the pyrrole units in the macrocycle, which is in agreement with the determined spatial distribution for the 160 mV feature. According to the conductance map presented in Fig. 3(e), the 240 mV vibrations appears to share the same origin as the 160 mV vibrations. However, the 80 mV-spaced Kondo replicas are a consequence of electron coupling to an 80 mV phonon. Lower energy vibrations are considered to be resulting from metal–ligand vibrations often mixed with porphyrin deformations.37–39
The presence of the electron–phonon coupling inside the molecule may also explain the lower TK measured for the in situ synthesized CoTPyP molecules. In general, the TK of Co-porphyrin derivates ranges between 100 K and 550 K10,11,20,40,41 as a function of the supporting substrate and of the functionalization of the porphyrin macrocycle. In particular, on a Au(111) substrate, CoTPP yield a TK around 225 K41 whereas chemically synthesized (ex situ) CoTPyP yield a TK in the range of 100–118 K when they are part of a molecular array. A recent theoretical investigation by Roura-Bas et al.22 of nonequilibrium transport through magnetic vibrating molecules led to the conclusion that the Kondo temperature will vary in the presence of electron–phonon coupling: a decrease in the TK is expected with increasing electron–phonon coupling due to renormalization of the hybridization.22 The lower TK measured for the in situ synthesized CoTPyP molecules plus the additional Kondo replicas present on both sides of the Fermi level suggest the presence of stronger electron–phonon interactions in this system than in the other molecular systems mentioned. The absence of electron–phonon coupling in CrTPyP molecules may be due to stronger molecule–substrate interactions that quenches the electron–phonon coupling and enhances the spin–electron coupling as observed previously for CrTPyP on Ag(111) surface.6 Use of complementary techniques like X-ray absorption spectroscopy and X-ray magnetic circular dichroism based measurements may reveal additional information on the magnetism of CoTPyP and CrTPyP and on their interaction with the underlying substrate. Moreover, future theoretical studies of such systems may not only help to further understand their properties but may also suggest new ways to experimentally tailor the magnetic properties of molecules by controlling the electron–phonon interactions.
Finally, we want to discuss the effect of the deposited adatom coverage on the molecular networks. The outcome of the magnetic-atom deposition onto the TPyP close-packed molecular structures is two fold and can be summarized as follows. On one hand, adatom coverages below one per molecule mostly lead to the creation of magnetic molecules as discussed in detail above. On the other hand, adatom coverages exceeding one adatom per molecule provide excess magnetic adatoms to decorate the molecular assembly at the hollow sites (intermolecular junctions) forming organo-metallic networks. Interestingly, the formation of such lattices is observed only for molecular patterns of type A indicating a preferential four-fold binding of Co (Cr) to pyridyl ligands. Careful inspection of high-resolution STM images like those presented in Fig. 1 can help discriminate between different occupation of the molecular junctions: single atom inserted at the four-pyridyl junction (marked by an arrow) can be easily distinguished from the empty junction or from the multi-atom features (marked by a dotted circle).21 Beside visual inspections of the structural properties, conductance measurements are also necessary to characterize these single- or multi-atom junctions.42 A description of the magnetic properties of these metallic junctions is presented in Section III of ESI.†
In conclusion, we probed electron–phonon interactions in synthesized magnetic metalloporphyrins using scanning tunneling spectroscopy and Kondo physics. The magnetic molecules were synthesized in situ via chemical reactions between TPyP molecules and magnetic atoms (Co and Cr). Our results show that both synthesized molecules develop a magnetic moment upon doping and their surface assemblies can be used as potential templates for realization of organometallic networks or periodic arrangement of metallic particles. However, electron–phonon interactions that lead to interplay between Kondo physics and vibrational excitations are observed only in CoTPyP molecules. Exploiting electron–phonon interactions in metallo-organic molecules may open up novel routes for tailoring their magnetic properties for use in molecular spintronics.
This work has been supported by the Research Foundation – Flanders (FWO, Belgium). Z. L. acknowledges the support from the China Scholarship Council (No. 2011624021) and from Internal KU Leuven funds.
References
- S. Sanvito, Chem. Soc. Rev., 2011, 40, 3336–3355 RSC.
- W. Auwärter, D. Ecija, F. Klappenberger and J. V. Barth, Nat. Chem., 2015, 7, 105 CrossRef PubMed.
- W. Auwärter, A. Weber-Bargioni, A. Riemann, A. Schiffrin, O. Gróning, R. Fasel and J. V. Barth, J. Chem. Phys., 2006, 124, 194708 CrossRef PubMed.
- F. Klappenberger, A. Weber-Bargioni, W. Auwärter, M. Marschall, A. Schiffrin and J. V. Barth, J. Chem. Phys., 2008, 129, 214702 CrossRef CAS PubMed.
- Z. Shi and N. Lin, J. Am. Chem. Soc., 2009, 131, 5376–5377 CrossRef CAS PubMed.
- K. Schouteden, Ts. Ivanova, Z. Li, V. Iancu, E. Janssens and C. Van Haesendonck, J. Phys. Chem. Lett., 2015, 6, 1048–1052 CrossRef CAS PubMed.
- Y. Li and N. Lin, Phys. Rev. B, 2011, 84, 125418 CrossRef.
- H. Kim, Y. H. Chang, S.-H. Lee, S. Lim, S.-K. Noh, Y.-H. Kim and S.-J. Kahng, Chem. Sci., 2014, 5, 2224–2229 RSC.
- W. Auwärter, A. Weber-Bargioni, S. Brink, A. Riemann, A. Schiffrin, M. Ruben and J. V. Barth, ChemPhysChem, 2007, 8, 250–254 CrossRef PubMed.
- A. Zhao, Q. Li, L. Chen, H. Xiang, W. Wang, S. Pan, B. Wang, X. Xiao, J. Yang, J. G. Hou and Q. Zhu, Science, 2005, 309, 1542–1544 CrossRef CAS PubMed.
- V. Iancu, A. Deshpande and S.-W. Hla, Nano Lett., 2006, 6, 820–823 CrossRef CAS PubMed.
- A. Mugarza, C. Krull, R. Robles, S. Stepanow, G. Ceballos and P. Gambardella, Nat. Commun., 2011, 2, 490 CrossRef PubMed.
- G. D. Scott and D. Natelson, ACS Nano, 2010, 4, 3560–3579 CrossRef CAS PubMed.
- T. Komeda, H. Isshiki, J. Liu, Y.-F. Zhang, N. Lorente, K. Katoh, B. K. Breedlove and M. Yamashita, Nat. Commun., 2011, 2, 217 CrossRef PubMed.
- J. Chen, M. A. Reed, A. M. Rawlett and J. M. Tour, Science, 1999, 286, 1550–1552 CrossRef CAS PubMed.
- N. P. Guisinger, M. E. Greene, R. Basu, A. S. Baluch and M. C. Hersam, Nano Lett., 2004, 4, 55–59 CrossRef CAS.
- R. P. Andres, T. Bein, M. Dorogi, S. Feng, J. I. Henderson, C. P. Kubiak, W. Mahoney, R. G. Osifchin and R. Reifenberger, Science, 1996, 272, 1323–1325 CAS.
- A. Riss, S. Wickenburg, L. Z. Tan, H.-Z. Tsai, Y. Kim, J. Lu, A. J. Bradley, M. M. Ugeda, K. L. Meaker, K. Watanabe, T. Taniguchi, A. Zettl, F. R. Fischer, S. G. Louie and M. F. Crommie, ACS Nano, 2014, 8, 5395–5401 CrossRef CAS PubMed.
- V. Iancu, K.-F. Braun, K. Schouteden and C. Van Haesendonck, Phys. Rev. Lett., 2014, 113, 106102 CrossRef PubMed.
- Q. Zhang, G. Kuang, R. Pang, X. Shi and N. Lin, ACS Nano, 2015, 9, 12521–12528 CrossRef CAS PubMed.
- T. Lin, G. Kuang, W. Wang and N. Lin, ACS Nano, 2014, 8, 8310–8316 CrossRef CAS PubMed.
- P. Roura-Bas, L. Tosi and A. A. Aligia, Phys. Rev. B, 2013, 87, 195136 CrossRef.
- All experiments are conducted in a UHV system (base pressure of 10–11 mbar) that includes an Omicron low temperature STM operated at 4.5 K.
- A detailed analysis on the dehydrogenation of TPyP macrocycle triggered by insertion of Co and Cr is given in ref. 6.
- V. Madhavan, W. Chen, T. Jamneala, M. F. Crommie and N. S. Wingreen, Science, 1998, 280, 567–569 CrossRef CAS PubMed.
- J. Li, W.-D. Schneider, R. Berndt and B. Delley, Phys. Rev. Lett., 1998, 80, 2893–2896 CrossRef CAS.
- J. Liu, H. Isshiki, K. Katoh, T. Morita, B. K. Breedlove, M. Yamashita and T. Komeda, J. Am. Chem. Soc., 2013, 135, 651 CrossRef CAS PubMed.
- U. Fano, Phys. Rev., 1961, 124, 1866–1878 CrossRef CAS.
- O. Újsághy, J. Kroha, L. Szunyogh and A. Zawadowski, Phys. Rev. Lett., 2000, 85, 2557–2560 CrossRef PubMed.
- These measurements are carried out only on molecules that are part of a square molecular network and therefore we do not comment on the effect that the network has on their magnetic properties as was presented recently in ref. 20 and 21.
- B. C. Stipe, M. A. Rezaei and W. Ho, Science, 1998, 280, 1732–1735 CrossRef CAS PubMed.
- N. B. Zhitenev, H. Meng and Z. Bao, Phys. Rev. Lett., 2002, 88, 226801 CrossRef CAS PubMed.
- Z.-Z. Chen, H. Lu, R. Lu and B. Zhu, J. Phys.: Condens. Matter, 2006, 18, 5435–5446 CrossRef CAS.
- I. Fernández-Torrente, K. J. Franke and J. I. Pascual, Phys. Rev. Lett., 2008, 101, 217203 CrossRef PubMed.
- A. Atamian, R. J. Donohoe, J. S. Lindsey and D. F. Bocian, J. Phys. Chem., 1989, 93, 2236–2243 CrossRef.
- Z. Zhang, S. Hou, Z. Zhu and Z. Liu, Langmuir, 2000, 16, 537–540 CrossRef CAS.
- K. F. Domke and B. Pettinger, ChemPhysChem, 2009, 10, 1794 CrossRef CAS PubMed.
- D. Li, Z. Peng, L. Deng, Y. Shen and Y. Zhou, Vib. Spectrosc., 2005, 39, 191–199 CrossRef CAS.
- J. Kincaid and K. Nakamoto, J. Inorg. Nucl. Chem., 1975, 37, 85–89 CrossRef CAS.
- A. Zhao, Z. Hu, B. Wang, X. Xiao, J. Yang and J. G. Hou, J. Chem. Phys., 2008, 128, 234705 CrossRef PubMed.
- H. Kim, Y. H. Chang, S.-H. Lee, Y.-H. Kim and S.-J. Kahng, ACS Nano, 2013, 7, 9312–9317 CrossRef CAS PubMed.
- T. Jamneala, V. Madhavan and M. F. Crommie, Phys. Rev. Lett., 2001, 87, 256804 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (I) TPyP adsorption on Au(111) and the metalation of porous structures; (II) additional scanning tunneling spectroscopy data for CoTPyP networks; (III) STS of metallic intermolecular junctions formed after Cr deposition. See DOI: 10.1039/c6cc03847f |
| This journal is © The Royal Society of Chemistry 2016 |