Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Active tumor-targeting luminescent gold clusters with efficient urinary excretion†
Xiaojuan
Wang
ab,
Hua
He
ab,
Yanan
Wang
b,
Junying
Wang
c,
Xing
Sun
 b,
Hai
Xu
ab,
Werner M.
Nau
*d,
Xiaodong
Zhang
b,
Hai
Xu
ab,
Werner M.
Nau
*d,
Xiaodong
Zhang
 *c and
Fang
Huang
*ab
*c and
Fang
Huang
*ab
aState Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, China. E-mail: fhuang@upc.edu.cn
bCentre for Bioengineering and Biotechnology, China University of Petroleum (East China), Qingdao 266580, China
cInstitute of Radiation Medicine and Tianjin Key Laboratory of Molecular Nuclear Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China. E-mail: xiaodongzhang@tju.edu.cn
dDepartment of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, 28759 Bremen, Germany. E-mail: w.nau@jacobs-university.de
First published on 17th June 2016
Abstract
We present novel active targeting luminescent gold nanoclusters (AuNCs), which are prepared through a one-pot procedure by using a pentapeptide (CRGDS) for stabilization and tumor recognition. CRGDS–AuNCs exhibit a high tumor-specific retention with an exceptionally high tumor-to-liver uptake ratio of 9.3. Their small hydrodynamic diameter and zwitterionic surface facilitate urinary excretion, which reaches 82% within 24 h after injection.
Tumor-targeting fluorescence imaging presents a fast, highly sensitive, and noninvasive method for tumor diagnosis; it stands out, in particular, in the detection of small lesions.1 In clinical practice, this method is being applied for the identification and localization of precancerous and cancerous tissues as well as for image-guided therapy.2
In order to achieve a good visualization of the tumor area, a wide range of fluorescent nanomaterials, including rare earth-containing upconversion nanoparticles,3,4 semiconductor quantum dots,5–7 carbon nanomaterials,8 and noble metal nanoclusters,9–13 have been explored as exogenous fluorophores. Among these, small gold nanoclusters (AuNCs) have attracted prime attention due to their ultra-small size, good biocompatibility, and extraordinary stability.10–16 While passive targeting AuNCs suffer invariably from low tumor specificity and the associated cytotoxic effects,11,13,17–20 the introduction of active tumor-targeting elements requires post-synthetic ligand modification. Unfortunately, tethering with recognition motifs leads to large hydrodynamic diameters (HDs) which in turn results in poor renal clearance as well as high in vivo toxicity – offsetting the original size advantages of AuNCs.5,21–30 The present challenge in medical diagnostics lies in the development of novel nanomaterials which display both high tumor specificity and efficient urinary excretion (see the ESI†). Herein, we present novel active tumor-targeting AuNCs, which meet these requirements.
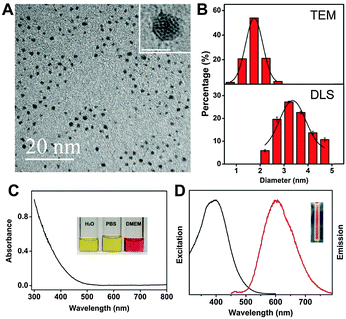
We used the pentapeptide Cys-Arg-Gly-Asp-Ser (CRGDS, Scheme 1) as the stabilizing and tumor-guiding ligand. This short peptide offers not only a side-chain thiol residue as an anchor for the Au clusters but also a binding domain (RGDS). The short peptide structure proved to be stable under the employed AuNC synthesis conditions, consistent with the subsequent characterization of the formed nanoclusters (size, distribution, surface charge) and the activity profiles (tumor cell targeting). RGDS recognizes αvβ3 integrins, which are over-expressed in both tumor and angiogenic endothelial cells.31–36 The novel one-pot synthetic strategy not only simplifies the preparation of active targeting AuNCs, but also ensures sufficiently small HDs to facilitate renal clearance and to retain low in vivo toxicity. Interestingly, although an internal cysteine residue has been considered crucial for stabilization through peptide modification,37 the attachment through the terminal cysteine residue afforded orange-emitting CRGDS–AuNCs which could be stored for 3 months without detectable changes in their dispersity and fluorescence properties (Fig. S1, ESI†). Their morphology and size distribution were characterized by high-resolution transmission electron microscopy (HRTEM) and dynamic light scattering (DLS, Fig. 1A and B). The results showed good dispersity and homogeneous size distribution. The average diameter of the gold cluster core was found to be 1.76 ± 0.26 nm by HRTEM, similar to the value reported for protein- and peptide-protected AuNCs.16,17,19,24,27,29 The HD value, which measures also the size of the protective bioorganic layer and which is most relevant for biocompatibility, was measured by DLS. The resulting value, 3.23 ± 0.30 nm, was indeed much smaller than the values obtained for previously fabricated active targeting AuNCs through postsynthetic ligand modification (6–120 nm).26–30 Most importantly, the HD value falls below the kidney filtration threshold of ca. 5.5 nm,12 a pre-requisite for clinical applications requiring efficient and rapid excretion, such as short-term exposure in the course of medical diagnosis.
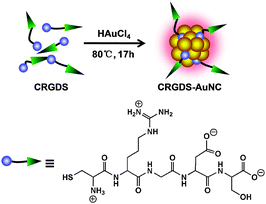 | ||
| Scheme 1 Direct synthesis of tumor-targeting AuNCs by using a multifunctional peptide for stabilization and tumor recognition. | ||
We used the pentapeptide recognition sequence CRGDS not only due to its small size but also due to its zwitterionic character, with a negative aspartate charge being balanced by a positive arginine one (Scheme 1). Nanoparticles carrying multiple cationic or anionic charges under physiological conditions – instead of being rapidly excreted – are known to bind to serum proteins, resulting in nonspecific accumulation in organs of the reticuloendothelial system (RES) and potential long-term toxicity.18 Expectedly, the zwitterionic character of the ligating peptide translated into an essentially neutral surface charge of CRGDS–AuNCs (a zeta potential of 0.2 ± 0.3 mV, measured in phosphate-buffered saline, PBS), which should further facilitate renal clearance and lower their potential RES toxicity.
Critical for the intended applications are the optical properties of CRGDS–AuNCs (Fig. 1C and D). The absorption spectra showed an onset at 500 nm but no surface plasmon resonance peak at 520 nm, as expected for small cluster sizes below 2 nm.38 The fluorescence emission spectra showed a maximum at 606 nm with a shoulder at 640 nm as well as a sizable quantum yield of 1.8%. CRGDS–AuNCs disperse well in water, PBS, and cell medium without obvious agglomeration. In regard to photostability, control experiments with the widely used fluorescein dye FITC (250-second continuous laser irradiation) showed that the fluorescence intensity of CRGDS–AuNCs remained above 80% of their initial value while that of FITC had already decreased sharply, below 30% (Fig. S2, ESI†).
With the pre-requisites for efficient renal clearance (small HD, neutral surface charge) and those for fluorescence detection (red emission, sizable quantum yield, and high photostability) being met, we proceeded to investigate the biocompatibility and biofunctionality. First, the cytotoxicity of CRGDS–AuNCs was evaluated by cell viability assays using HeLa and MCF-7 cells (Fig. S5, ESI†). Since their proliferation remained at a constant level after 12 h or 24 h incubation, even at very high CRGDS–AuNC concentrations (up to 500 μg mL−1), no indications of significant cytotoxicity could be obtained.
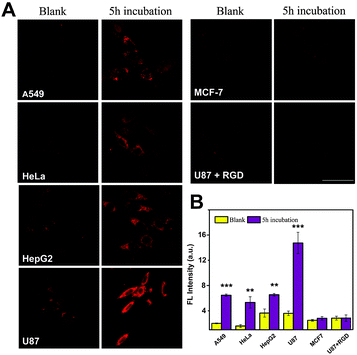
The active targeting properties of CRGDS–AuNCs as well as their specificity were studied by confocal microscopy using five different cell lines. As shown in Fig. 2, after incubation with CRGDS–AuNCs (300 μg mL−1) for 5 h, all four αvβ3 integrin-positive tumor cell lines (A549, HeLa, HepG-2, and U87) displayed bright fluorescence signals, whereas no significant signal was observed in the αvβ3 integrin-negative cell line (MCF-7). The qualitatively different response immediately confirmed the specific recognition ability at the cellular level and indicated an active rather than a passive targeting pathway.
The magnified images of U87 and HepG2 cells revealed a typical endocytotic localization of CRGDS–AuNCs in the cell cytoplasm (Fig. S4, ESI†). The quantitative fluorescence analysis of HeLa (+) and MCF-7 (−) cells after different incubation times also revealed that the signal in MCF-7 cells remained negligible, while that in HeLa increased strongly with time (Fig. S3, ESI†), which is again consistent with an enhanced cellular uptake through an active targeting mechanism. Active uptake was corroborated through a competition experiment in which the staining by CRGDS–AuNCs was followed in the presence of excess free CRGDS peptide as a competitor for αvβ3 integrin in U87 (+) cells. Only in the absence of the free peptide did the cells produce the desired fluorescence signal, which demonstrates the competition of both – free and ligated – pentapeptide sequences for the same recognition site: αvβ3 integrin. Accordingly, CRGDS–AuNCs actively target tumor cells through αvβ3 integrin-mediated endocytosis.
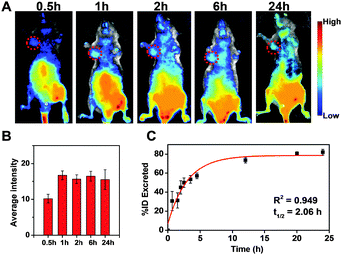
In view of the low cytotoxicity and favorable active tumor-targeting properties, we proceeded to in vivo imaging experiments with CRGDS–AuNCs in HeLa and U87 tumor-bearing nude male mice. As shown in Fig. 3A, B and Fig. S6 (ESI†) strong fluorescence emission was observed at the tumor site (indicated by circles) just 30 min after injection. Within the next 6 hours, the fluorescence intensity increased gradually and the tumor area became better defined. Subsequently, the signal remained relatively invariant at a high level for ca. 24 h, while strong fluorescence was also readily observed in the bladder, indicating rapid renal clearance of the AuNCs. The urinary excretion profile of CRGDS–AuNCs was further investigated in C57 mice. The fluorescence of AuNCs appeared in the urine just 40 min after injection. The total number of excreted CRGDS–AuNCs was calculated as the sum of the fluorescent components in the urine and was fit with an exponential curve to determine the kinetics of renal clearance (Fig. 3C).17,39,40 We found a urine half-life of ca. 2 h in C57 mice, that is, more than 80% of the CRGDS–AuNCs were excreted within 24 h. This efficient urinary clearance profile is in good agreement with the renal filtration threshold reported for extremely small inorganic, metal-containing nanoparticles.41
The observed tumor retention times of up to 24 h and the efficient renal clearance are ideal for potential clinical applications.17 Until now, only low molecular-weight probes can compete with small AuNCs in terms of their renal clearance efficiency, but the integrin-binding ability of CRGDS–AuNCs prolongs their tumor retention time, as desired.
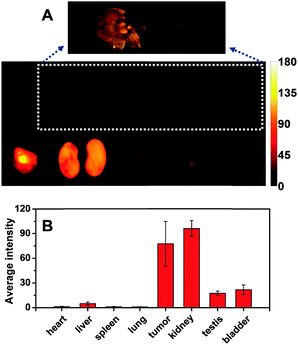
The bio-distribution of CRGDS–AuNCs in vital organs and tumor tissue removed 24 h after intravenous injection was characterized by ex vivo imaging. As shown in Fig. 4A, a very strong fluorescence signal is found in the tumor tissue, indicating that CRGDS–AuNCs have significant targeting capability in the systemic circulation for the deposition in the tumor. Meanwhile, the kidney also afforded a very strong signal, fully in line with the highly efficient renal clearance. The CRGDS–AuNC uptake into other vital organs was much less (Fig. 4B), which is consistent with the in vivo imaging results. It is important to note that the liver shows a negligible fluorescence signal, which suggests that CRGDS–AuNCs can by-pass the first-pass extraction from the RES as a consequence of their efficient urinary excretion. Strikingly, the tumor-to-liver uptake ratio of CRGDS–AuNCs 24 h after intravenous injection was found to be 9.3, much higher than the values previously reported for tumor-targeting AuNCs (range of 1–3, both for passive targeting and active targeting).11,13,17–20,26–30 Experiments with U87 tumor-bearing nude mice showed that even 48 h after intravenous injection, the tumor-to-liver uptake ratio of CRGDS–AuNCs was still as high as 3.1 (Fig. S7, ESI†). These experimental findings confirm the synergistic improvement of efficient tumor targeting and low liver accumulation of CRGDS–AuNCs, underlining the diagnostic potential of the newly designed CRGDS–AuNCs.
In conclusion, we have obtained proof-of-concept that a simple pentapeptide, CRGDS, can bestow a beneficial combination of functions and properties onto AuNCs. It not only stabilizes the luminescent AuNCs during circulation and enables them to be specifically taken up by the tumor cells, but also facilitates an efficient renal clearance of the resulting AuNCs, owing to their small HD and low surface charge. Their facile one-step synthesis, the exceptionally high tumor-to-liver uptake ratio, low accumulation in non-targeted cells and tissues, their low cytotoxicity, long tumor retention time, and fast renal clearance rates render CRGDS–AuNCs novel and promising probes for tumor-targeting fluorescence imaging. Follow-up biological studies, including in vivo toxicity assays and tumor penetration profiles by ICP-MS in different tumor tissues, are planned in order to proceed towards clinical tumor diagnosis.
This study was supported by the National Key Basic Research Program of China (2012CB518000), the National Natural Science Foundation of China (21103230, 21033005), the Natural Science Foundation of Shandong Province (ZR2014BM028), and the DFG.
Notes and references
- A. Sieroń, K. Sieroń-Stołtny, A. Kawczyk-Krupka, W. Latos, S. Kwiatek, D. Straszak and A. M. Bugaj, OncoTargets Ther., 2013, 6, 977–982 Search PubMed.
- K. Moghissi, M. R. Stringer and K. Dixon, Photodiagn. Photodyn. Ther., 2008, 5, 235–237 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- H. H. Gorris and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 3584–3600 CrossRef CAS PubMed.
- C. Zhang, X. Ji, Y. Zhang, G. Zhou, X. Ke, H. Wang, P. Tinnefeld and Z. He, Anal. Chem., 2013, 85, 5843–5849 CrossRef CAS PubMed.
- Y. He, Y. Zhong, Y. Su, Y. Lu, Z. Jiang, F. Peng, T. Xu, S. Su, Q. Huang and C. Fan, Angew. Chem., Int. Ed., 2011, 50, 5695–5698 CrossRef CAS PubMed.
- Y. Park, Y.-M. Ryu, Y. Jung, T. Wang, Y. Baek, Y. Yoon, S. M. Bae, J. Park, S. Hwang, J. Kim, E.-J. Do, S.-Y. Kim, E. Chung, K. H. Kim, S. Kim and S.-J. Myung, ACS Nano, 2014, 8, 8896–8910 CrossRef CAS PubMed.
- X. Wang, X. Sun, J. Lao, H. He, T. Cheng, M. Wang, S. Wang and F. Huang, Colloids Surf., B, 2014, 122, 638–644 CrossRef CAS PubMed.
- J. Wang, J. Ye, H. Jiang, S. Gao, W. Ge, Y. Chen, C. Liu, C. Amatore and X. Wang, RSC Adv., 2014, 4, 37790–37795 RSC.
- H. Qian, M. Zhu, Z. Wu and R. Jin, Acc. Chem. Res., 2012, 45, 1470–1479 CrossRef CAS PubMed.
- X. Wu, X. He, K. Wang, C. Xie, B. Zhou and Z. Qing, Nanoscale, 2010, 2, 2244–2249 RSC.
- Z. Luo, K. Zheng and J. Xie, Chem. Commun., 2014, 50, 5143–5155 RSC.
- X.-D. Zhang, Z. Luo, J. Chen, X. Shen, S. Song, Y. Sun, S. Fan, F. Fan, D. T. Leong and J. Xie, Adv. Mater., 2014, 26, 4565–4568 CrossRef CAS PubMed.
- H. Maeda, Adv. Enzyme Regul., 2001, 41, 189–207 CrossRef CAS PubMed.
- K. Yang, J. Wan, S. Zhang, B. Tian, Y. Zhang and Z. Liu, Biomaterials, 2012, 33, 2206–2214 CrossRef CAS PubMed.
- X.-D. Zhang, J. Chen, Z. Luo, D. Wu, X. Shen, S.-S. Song, Y.-M. Sun, P.-X. Liu, J. Zhao, S. Huo, S. Fan, F. Fan, X.-J. Liang and J. Xie, Adv. Healthcare Mater., 2014, 3, 133–141 CrossRef CAS PubMed.
- J. Liu, M. Yu, C. Zhou, S. Yang, X. Ning and J. Zheng, J. Am. Chem. Soc., 2013, 135, 4978–4981 CrossRef CAS PubMed.
- C. Zhou, M. Long, Y. Qin, X. Sun and J. Zheng, Angew. Chem., Int. Ed., 2011, 50, 3168–3172 CrossRef CAS PubMed.
- C. Zhou, G. Hao, P. Thomas, J. Liu, M. Yu, S. Sun, O. K. Öz, X. Sun and J. Zheng, Angew. Chem., Int. Ed., 2012, 51, 10118–10122 CrossRef CAS PubMed.
- P. Huang, J. Lin, S. Wang, Z. Zhou, Z. Li, Z. Wang, C. Zhang, X. Yue, G. Niu, M. Yang, D. Cui and X. Chen, Biomaterials, 2013, 34, 4643–4654 CrossRef CAS PubMed.
- Y. L. Yang, F. F. An, Z. Liu, X. J. Zhang, M. J. Zhou, W. Li, X. J. Hao, C. S. Lee and X. H. Zhang, Biomaterials, 2012, 33, 7803–7809 CrossRef CAS PubMed.
- D. Yang, X. Kang, P. Ma, Y. Dai, Z. Hou, Z. Cheng, C. Li and J. Lin, Biomaterials, 2013, 34, 1601–1612 CrossRef CAS PubMed.
- G. Gu, H. Xia, Q. Hu, Z. Liu, M. Jiang, T. Kang, D. Miao, Y. Tu, Z. Pang, Q. Song, L. Yao, H. Chen, X. Gao and J. Chen, Biomaterials, 2013, 34, 196–208 CrossRef CAS PubMed.
- Y. Wang, Y. Cui, Y. Zhao, R. Liu, Z. Sun, W. Li and X. Gao, Chem. Commun., 2012, 48, 871–873 RSC.
- L. Li, J. Hou, X. Liu, Y. Guo, Y. Wu, L. Zhang and Z. Yang, Biomaterials, 2014, 35, 3840–3850 CrossRef CAS PubMed.
- D. Chen, Z. Luo, N. Li, J. Y. Lee, J. Xie and J. Lu, Adv. Funct. Mater., 2013, 23, 4324–4331 CrossRef CAS.
- C. Zhang, C. Li, Y. Liu, J. Zhang, C. Bao, S. Liang, Q. Wang, Y. Yang, H. Fu, K. Wang and D. Cui, Adv. Funct. Mater., 2015, 25, 1314–1325 CrossRef CAS.
- A. Retnakumari, S. Setua, D. Menon, P. Ravindran, H. Muhammed, T. Pradeep, S. Nair and M. Koyakutty, Nanotechnology, 2010, 21, 055103 CrossRef PubMed.
- J. Qiao, X. Mu, L. Qi, J. Deng and L. Mao, Chem. Commun., 2013, 49, 8030–8032 RSC.
- H. Chen, S. Li, B. Li, X. Ren, S. Li, D. M. Mahounga, S. Cui, Y. Gu and S. Achilefu, Nanoscale, 2012, 4, 6050–6064 RSC.
- J. S. Desgrosellier and D. A. Cheresh, Nat. Rev. Cancer, 2010, 10, 9–22 CrossRef CAS PubMed.
- J. O. Winter, T. Y. Liu, B. A. Korgel and C. E. Schmidt, Adv. Mater., 2001, 13, 1673–1677 CrossRef CAS.
- B. S. Shah, P. A. Clark, E. K. Moioli, M. A. Stroscio and J. J. Mao, Nano Lett., 2007, 7, 3071–3079 CrossRef CAS PubMed.
- A. Kumar, H. Ma, X. Zhang, K. Huang, S. Jin, J. Liu, T. Wei, W. Cao, G. Zou and X.-J. Liang, Biomaterials, 2012, 33, 1180–1189 CrossRef CAS PubMed.
- Y. J. Cheng, G. F. Luo, J. Y. Zhu, X. D. Xu, X. Zeng, D. B. Cheng, Y. M. Li, Y. Wu, X. Z. Zhang, R. X. Zhuo and F. He, ACS Appl. Mater. Interfaces, 2015, 7, 9078–9087 Search PubMed.
- K. Sarkar, S. L. Banerjee, P. P. Kundu, G. Madras and K. Chatterjee, J. Mater. Chem. B, 2015, 3, 5266–5276 RSC.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- F. Mafuné, Chem. Phys. Lett., 2004, 397, 133–137 CrossRef.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed.
- A. A. Burns, J. Vider, H. Ow, E. Herz, O. Penate-Medina, M. Baumgart, S. M. Larson, U. Wiesner and M. Bradbury, Nano Lett., 2009, 9, 442–448 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, supporting text and Fig. S1–S7. See DOI: 10.1039/c6cc03814j |
| This journal is © The Royal Society of Chemistry 2016 |