Open Access Article
Open Access ArticleReactions in ultra-small droplets by tip-assisted chemistry
M.
Guardingo†
*ab,
F.
Busqué
b and
D.
Ruiz-Molina
*a
aCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC and The Barcelona Institute of Science and Technology, Campus UAB, Bellaterra 08193, Barcelona, Spain. E-mail: mireia.guardingo@gmail.com; dani.ruiz@icn2.cat
bDepartament de Química, Universitat Autònoma de Barcelona (UAB), Campus UAB, Cerdanyola del Vallès 08193, Barcelona, Spain
First published on 19th July 2016
Abstract
The confinement of chemical reactions within small droplets has received much attention in the last few years. This approach has been proved successful for the in-depth study of naturally occurring chemical processes as well as for the synthesis of different sets of nanomaterials with control over their size, shape and properties. Different approaches such as the use of self-contained structures or microfluidic generated droplets have been followed over the years with success. However, novel approaches have emerged during the last years based on the deposition of femtolitre-sized droplets on surfaces using tip-assisted lithographic methods. In this feature article, we review the advances made towards the use of these ultra-small droplets patterned on surfaces as confined nano-reactors.
Introduction
Femtolitre chemistry has emerged in the last few years as an exciting approach to synthesize nanoscale materials in a highly controlled manner. In combination with lithographic and micro/nano-fabrication techniques, it has opened the door to the creation of large and dense arrays of nano-reaction vessels for high-throughput screening, combinatorial chemistry/biology or chemical synthesis.1 Beyond the need for nanostructured materials, there are several other scientific motivations to conduct chemistry at this scale. A femtolitre (fL = 10−15 L, 1 μm3) is approximately the volume of a bacterial cell, and the ultimate chemistry of life takes place at this ultra-small scale that ranges from picolitres (pL = 10−12 L, 10 μm3) to attolitres (aL = 10−18 L, 100 nm3).2 Reproducing these highly crowded and confined conditions is therefore essential to understand their effect on the thermodynamics and kinetics of confined biological and chemical reactions. This need has fuelled the development of a wide range of synthetic nanostructured (bio)environments, including cell-like compartments for encapsulating biochemical reactions, nanostructured containers for fundamental studies of diffusion, or nanofabricated topological features that regulate biomolecular interactions.3 In addition, the study of naturally occurring chemical processes under confined conditions may shed light onto relevant fields such as the origin of life or atmospheric aerosols that are still poorly understood.4Apart from the fundamental studies of (bio)chemical processes at the nanoscale, applied chemistry has also benefited from the advances made in femtolitre chemistry. In the synthesis of nanomaterials, droplets can act as templates to control parameters such as particle size and shape or surface texture5 and thus, to tune morphology–size–property relationships. So far, different approaches have been followed to generate miniaturized droplet-based reactors. The most extended procedures make use of self-contained structures (like droplet emulsions, liposomes, micelles and protein cages) or microfluidic-generated droplets. An alternative methodology consisting in depositing small droplets on a surface using tip-assisted lithographic methods has emerged in the last few years. Using this approach, the droplets can be used as confined reactors to precisely control the position of the resulting materials on the substrate. The interest of this methodology lies in the reduction of the number of steps needed to pattern functional materials on a surface, as the synthesis and patterning processes are performed simultaneously.
In this feature article, we review recent research involving femtolitre-sized reactions. The methodologies based on self-contained structures and microfluidic-generated droplets have already been extensively reviewed2,6,7 and are only briefly addressed here. Instead, we mainly concentrate on the emerging tip-assisted methodologies and the materials obtained directly in femtolitre-sized droplets deposited on surfaces.
Confined reactions in self-contained structures and microfluidic channels
Reactions confined in self-assembled containers
Water-in-oil emulsions are metastable colloids that represent the simplest example of nanocontainers. They are composed of two immiscible fluids, one being dispersed in the other in the shape of femtolitre-sized droplets.8 These structures have been extensively used to confine biochemical reactions such as the polymerase chain reaction (PCR)9 and other processes like cell-free protein expression.10 The resemblance of the lipid bi-layer wall of liposomes to cell membranes has favoured their consideration as “artificial cells”11 and arrays of lipid vesicles have been suggested as libraries for the simultaneous screening of multiple analytes.12 Moreover, the permeability and stability of the lipid bilayer can be tuned through external stimuli such as electric pulses or temperature changes to trigger the reactions occurring inside the liposomes.13,14 The use of capsosomes, liposomes embedded within polymeric capsules, allows coupled and parallel enzymatic reactions to be performed under confined conditions by loading the liposomes with different enzymes.15These supramolecular organic templates have also been extensively used for the synthesis of multiple nanoscale solids, from metallic and ceramic nanoparticles5,16–20 to hybrid composites21 or metal–organic particles.22 The strategy consists of mixing two microemulsions (direct or reverse), one containing the metallic precursor and the other one the so-called precipitating agent (Fig. 1). Among all the different nanomaterials synthesized in this way, nanoscale metal–organic materials and coordination polymers have especially benefitted from this methodology, as it allows radical improvement of the control over the size, shape and crystallinity of the resulting particles.23 Indeed, since the pioneering work by Mann and co-workers on the synthesis of Prussian blue nanoparticles in reverse microemulsions,24 an increasing amount of reports have appeared that employ this method to obtain and control the shape and size of Prussian blue analogs,25–28 metal–organic frameworks (MOFs),29–32 or spin-crossover polymers33–36 among others. Microemulsions have also been used as nanoreactors to produce polymeric nanoparticles37–40 and protein nanoparticles.41,42
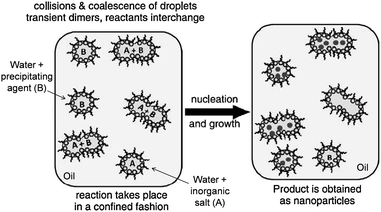 | ||
| Fig. 1 Schematic representation of the synthesis of nanoparticles using water-in-oil emulsions. For purely inorganic materials, the precipitating agent (B) is usually a reducing agent such as NaBH4; in the case of metal–organic particles, (B) corresponds to the organic ligand(s). Reproduced with permission from ref. 23. | ||
Beyond synthetic assemblies, natural nanoarchitectures such as viral capsids and other protein cages have also been used as nanoreactors.7,43,44 The main advantage of the use of natural nano-containers in comparison to synthetic supramolecular assemblies lies in their improved monodispersity and robustness as well as the broad range of sizes available and the possibility to easily functionalize the protein shell.45,46 For these reasons, protein-based nanocontainers have been used as templates for the synthesis of nanoscale inorganic materials such as metal and metal-oxide nanoclusters,47,48 as well as to perform and study confined enzymatic reactions.49–51 Self-assembling peptide polynanoreactors have also been described and applied to the synthesis of silver nanoparticles.52
Reactions confined in droplets generated in microfluidic channels and micro/nano-wells
Microfluidic devices are commonly used to generate and mix droplets under highly controlled environments. This high level of control is achieved thanks to the generation of microfluidic droplets with perfectly controlled and uniform size at the cross-stream flow of two immiscible liquids.6,53 Due to that, microfluidic generated droplets are highly reproducible synthetic environments that, in turn, provide highly reproducible conditions and materials. An important added value of performing femtolitre chemistry in microfluidic devices is the reduced amount of reagents and solvents that are consumed. This is highly important when performing screening studies using precious materials. For this reason, crystallization of proteins and pharmaceuticals54–56 as well as screening of organic synthetic reactions has been performed using microfluidic tools.57–59Plenty of examples on the use of microfluidics for confined biochemical reactions can be found in the literature, going from enzyme kinetics60 to protein expression61,62 and single-cell studies.63,64 However, probably the area in which this approach has offered more innovative advances is the synthesis of micro-/nanoparticles,65 as it allows for a precise control of the size distribution.66,67 As an example, gold nanoparticles68 or nanorods with tunable aspect ratios were obtained in microfluidics-generated droplets.69 Other technologically relevant inorganic nanocrystals such as CdSe quantum dots,70 superparamagnetic iron oxide nanoparticles (SPIONs),71 silver,72 zeolites73 or core–shell nanostructures74 as well as polymeric microcapsules75 and solid particles76 have also been obtained in this way. In addition, the confined synthesis of a series of MOFs and core–shell MOF composites using microfluidic tools has been recently described in two almost simultaneously released papers (Fig. 2).77,78 Both research groups reported that the confinement of the reaction afforded highly homogeneous crystals whilst significantly reducing the reaction time.79
 | ||
| Fig. 2 Optical and SEM micrographs of HKUST-1 crystals obtained in microfluidic droplets with increasing reaction times. Reproduced with permission from ref. 77. | ||
Finally, in parallel with microfluidics, homogeneous arrays of nanocontainers fabricated on surfaces using lithographic techniques or optical fiber bundles have been used to obtain large arrays of ultra-small reaction vessels.80–82 One of the most important achievements in this field has been the fabrication of so-called zero-mode waveguides,83 which have allowed the observation of single-molecule dynamics of increasingly complex biological systems at high concentrations.3
Tip-assisted lithography: an introduction
Direct-write AFM-assisted lithography (also referred to as scanning probe lithography (SPL), AFM lithography or tip-assisted lithography) is a high-resolution lithographic technique that uses a sharp tip to pattern nano-to-microscale features on a surface. It resembles a normal writing process where the AFM tip is used as a “pen”, a solid state substrate acts as “paper” and a solution containing the material(s) as an “ink”.84 The molecules or nanostructures acting as inks are first coated on the tip and then transported to the surface by engaging and traversing the tip over the substrate in the form of the desired pattern. Although any AFM probe can be theoretically employed, typically specially designed probes (commonly referred to as “pens”) are used, which show pyramidal shapes and tip radii of ∼15 nm.The process of transferring molecules from an AFM tip to a substrate was first described in 1995 by Jaschke and Butt,85 who deposited aggregates of octadecanethiol (ODT) in irregular-shaped structures with a homogeneous height of 1.2 nm onto freshly cleaved mica. A few years later, in 1999, Mirkin and co-workers organized alkanethiol molecules on Au surfaces forming well-defined SAMs with excellent resolution (down to 12 nm)86,87 and obtained multi-component patterns composed of different alkanethiols, reducing the separation to only 5 nm.88,89 These results led to the invention of a commercialized process called Dip-Pen Nanolithography® (abbreviated as DPN®) that became a registered trademark of NanoInk, Inc. (Chicago, IL). In the following years, the technique became increasingly popular90,91 and, since then, a myriad of materials have been successfully structured in a wide variety of substrates using DPN.92–104
The scalability of AFM-assisted lithography has always been questioned due to the low throughput of the technique, motivated by its inherent serial writing nature. Because of that, it has been considered a technique restricted to proof-of-concept studies and basic science. In order to expand the limits of the technique, other derived tip-assisted lithographic techniques such as polymer pen lithography (PPL) have appeared.105 This young technique combines the feature size control of direct-write AFM lithography with the large-area printing capability of micro-contact printing. The writing tool consists of an array of up to 11 million elastomeric pyramids (typically polydimethylsiloxane, PDMS) that are coated with the inks and brought into contact with the surface to create patterns over large areas.106–109 The appearance of this and other related massively parallel cantilever-free printing tools110–112 has solved the main inconvenience of direct-write AFM assisted lithography by turning it into a parallelized process.
On the other hand, the main advantage of direct-write AFM-assited lithography in comparison with other structuration techniques is that it allows for the precise positioning of materials under environmental conditions onto virtually any substrate without the need of prior surface or material modification.101 Due to that, this technique has been highly valued for patterning biological entities such as proteins,113–117 oligonucleotides118 or living cells.119 Moreover, it is a non-destructive technique that can be used on fragile and soft surfaces like polymers,120 graphene121–123 or living tissues.99,124,125 Also, direct-write AFM-assisted lithography is ideal to be used in fabrication processes of small devices where the last step consists of positioning valuable functional materials on specific areas of a solid support. For instance, our group deposited a diversity of magnetic materials (ferritin-based CoO nanoparticles, Mn12 single-molecule magnets and Co nanoparticles) on the most sensitive areas (as small as 1 μm2) of superconductive sensors to enhance their sensitivity without damaging any of the components of the devices.123,126–129
According to the ink's nature, direct-write AFM lithography experiments can be categorized into two main types: dry and liquid.102 In the classic (dry) methodology (Fig. 3a), the AFM tip is functionalized by small molecules that are transported to the substrate by diffusion through the water meniscus that is formed due to capillary condensation under ambient conditions.130,131 This procedure was originally developed for the deposition of alkanethiols,103 but it has been extended to more complex inks such as nanoparticles,132 biomolecules117,133 and materials supported in matrix carriers.111
 | ||
| Fig. 3 Schematic representation of the writing process in AFM-assisted lithography. (a) Schematics of the classic (dry) procedure in which a soluble small molecule ink diffuses through the water meniscus that forms at the point of contact. (b) Representation of the deposition of femtolitre-sized droplets of a solution directly on the surface. Reproduced with permission from ref. 135. | ||
A completely different methodology consists of dipping the tip in an ink solution for a given time and immediately using it before the solvent evaporates.134 In this case, the ink is patterned on the substrate by delivering less than femtoliter droplets of the solution (Fig. 3b). After patterning, the solvent evaporates and motifs of the materials in the solid state are obtained. The origin of this methodology lies in the need to pattern materials and nano-objects that do not diffuse easily (or do no diffuse at all) through the water meniscus. However, in the recent years some researchers have envisaged the possibility of using femtolitre-sized droplets deposited on surfaces as miniaturized vessels where reactions can be performed on an extremely small scale. Although this methodology is only in its infancy stages, several examples can be already found in the literature, as summarized next.
Chemistry within femtolitre droplets deposited by tip-assisted lithography
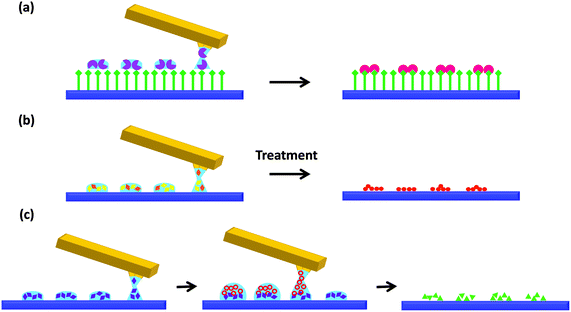
In the following section, we thoroughly review the existing publications on the use of tip-assisted lithographic methods to perform confined reactions on surfaces. For the sake of simplicity, we have classified the chemistry within femtolitre droplets deposited on surfaces into three different categories, as represented in Fig. 4: (I) reaction between the ink components and the substrate (Fig. 4a), (II) reaction of the components already contained in the droplets and delivered in a single step (Fig. 4b) and (III) mixture of reagents on a surface by sequential delivery of solutions containing the different reagents (Fig. 4c). The approaches and examples of each one of them are described in detail next.Approach I: reaction between materials contained in delivered droplets and the target surface
Out of the three, this is the approach for which a larger amount of examples have been described. It consists of the transformation of the delivered materials upon reaction with some specific groups on the surface. One strategy uses the redox properties of the substrate to in situ transform the patterned materials and obtain metallic nanostructures.For example, in 2001136 the reducing capability of an activated Si substrate was used to reduce Au(III) precursors deposited through an AFM tip and form metallic gold patterns. Later on, it was demonstrated that Au and Pt motifs could be obtained by using the same process on untreated Ge(100) substrates.137 More recently, the reducing capability of single-walled carbon nanotubes (SWCNTs) was used to deposit gold seeds on their surface by drawing lines over them with an AFM tip coated with HAuCl4.138 Although very useful, the applicability of this method is restricted to noble metal precursors that are easily reduced and the use of compatible substrates.
In contrast, a fairly common example of tip-mediated localized reaction consists of the reaction of at least one of the ink components with appropriate functionalities present on the substrate to form a covalent bond. This methodology is mainly employed to anchor functional compounds to the surface, and therefore the patterns can resist subsequent washing steps and wet treatments. The first example of this methodology was reported in 2007 and described the anchoring of an azide-functionalized dendron on an alkyne-modified surface139 through copper-catalyzed azide–alkyne cycloaddition (CuAAC, one of the most common examples of click-chemistry). Following this pioneering work, other reports have appeared employing the same methodology to pattern multiple substrates140 and a variety of materials, including fluorescent probes or biologically active species.141 For instance, our group used an amino-terminated surface to covalently anchor and pattern three fluorescent pH-responsive compounds (Oregon Green®, fluorescein and 5-carboxynaphthofluorescein) bearing amino-reactive groups. By using multiple cantilever arrays, large ordered and combinatorial arrays of these optically active molecules were fabricated.142 Exposure of the patterned surfaces to solutions or gas flows of different pH values resulted in reversible changes in the fluorescence signal of the patterned chemosensors (Fig. 5).
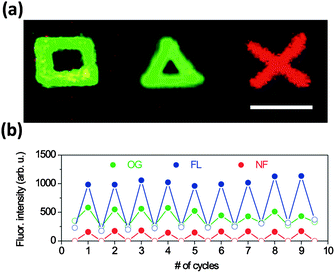 | ||
| Fig. 5 Acid–base reactions of optically active compounds structured on surfaces. (a) Fluorescence image of Oregon green (square), fluorescein (triangle) and 5-carboxynaphthofluorescein (cross) structures; scale bar 5 μm. (b) Average fluorescence intensity of Oregon green, fluorescein and 5-carboxynaphthofluorescein dots upon consecutive cycles of exposure to buffer solutions of pH 3.5 (empty circles) and 9.5 (full circles). Reproduced from ref. 142. | ||
Of course, massively parallel techniques derived from AFM-assisted lithography have also been used to fabricate femtolitre-sized reactors on a surface. For instance, Braunschweig et al. reported the use of polymer pen lithography (PPL) for copper-catalyzed click chemistry143 as well as for the Staudinger ligation144 to obtain fluorescent (Fig. 6) and redox-active patterns and protein recognition platforms anchored to different substrates. More recently, the same researchers used PPL to carry out force-accelerated Diels–Alder reactions on graphene sheets145and studied the effect of the applied force on the velocity of a 1,3-dipolar cycloaddition in the absence of copper.146 Another massively parallel tip-based technique derived from PPL, namely beam pen lithography (BPL),147 was used to produce 3D patterns of polymer brushes by photoinduced radical polymerization.148 On this occasion, the reaction performed inside the fabricated features not only involved the anchoring of the material to the surface but also the polymerization of the brushes.
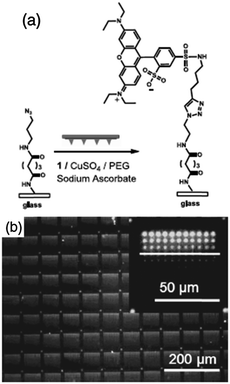 | ||
| Fig. 6 Fluorescence patterns produced by the site-specific copper-catalyzed azide–alkyne cycloaddition. (a) An ink mixture consisting of a fluorescent reagent, PEG, CuSO4, and sodium ascorbate printed onto an azido-terminated glass slide resulted in covalent immobilization of rhodamine. (b) Fluorescence microscopy image of 11 × 11 dot arrays obtained after patterning the fluorescent reagent with varying dwell times. The inset is a magnified image of one array. Reproduced with permission from ref. 143. | ||
Approach II: reaction between materials contained in femtolitre droplets
In this section, we address the cases in which femtolitre droplets containing reaction precursors are patterned on surfaces in order to force a reaction to proceed inside the deposited nanoreactors. In some cases, the reaction is triggered after patterning by an external stimulus, whilst in others it occurs spontaneously.The precedents to this approach were already settled a few years ago, when our group carried out the assembly and crystallization process of various metal–organic nanostructures confined in femtolitre droplets.149 Onn that occasion, crystals of well-known HKUST-1 ([Cu3(BTC)2], BTC = benzenedicarboxylate) and hollow structures of polyoxometalates (POMs) were grown directly on gold surfaces after delivering ultra-small droplets of the soluble precursors to the surface through an AFM tip (Fig. 7). A reduction in the volume of the deposited droplets, together with the precise control of solvent evaporation afforded the formation of a single nanostructure per deposited droplet.
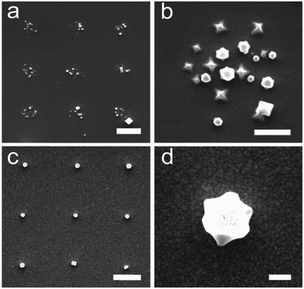 | ||
| Fig. 7 FE-SEM images of HKUST-1 nanocrystals grown inside confined solution droplets deposited by direct-write AFM-assisted lithography. (a) Nanoarray; scale bar 2 μm. (b) Nanocrystals grown inside each dot-like feature; scale bar 1 μm. Growth of a single crystal per dot nanoarray viewed from above (c) and at a 45° tilt angle (d); scale bars 2 μm and 200 nm, respectively. Reproduced from ref. 149. | ||
Almost simultaneously, Carbonell and co-workers also described the formation of single HKUST-1 crystals inside femtolitre-sized droplets that were fabricated using microfluidic pen lithography (MPL). This technique uses a microfluidic pen instead of an AFM tip to deliver a solution from a reservoir onto the surface.150,151 This overcomes one of the main disadvantages of direct-write AFM-assisted lithography, which is the depletion of the ink loaded on the cantilever, but at the same time larger droplets are deposited on the surface.
Beyond crystallization and self-assembly processes, the literature provides a fairly wide range of publications that report the performance of chemical reactions on a surface after the delivery of a mixture of reagents through an AFM tip. A diversity of metal oxides and sulfides have been obtained using this methodology. For example, nanostructures of Al2O3, SiO2 and SnO2 were fabricated on Si and SiO2 surfaces using sol-based inks.152 Briefly, chloride precursors of the metal oxides were brought onto the surface and spontaneously hydrolyzed after getting in contact with the water condensed at the meniscus. In another example, a mixture of Cd(CH3COO)2 and thioacetamide was delivered onto Si surfaces. Thioacetamide gradually releases H2S upon contact with water and thus CdS spontaneously forms after the ink diffuses to the substrate through the water meniscus.153 An analogous methodology was employed later on to grow CdS nanoplates on mica.154 In another example, a heat treatment was used to fabricate barium hexaferrite (BaFe12O19) magnetic nanostructures after the delivery of a mixture of Fe(NO3)3 and BaCO3 in ethylene glycol on a silicon oxide surface.155
The controlled growth of metallic and semiconductor nanoparticles directly on a surface has also received much attention. This is really not surprising given the versatility and interesting properties of these nanoparticles and the vast amount of applications that are derived from their assembly and patterning on surfaces.156–160 Since 2010, the Mirkin group has released several papers using scanning probe block copolymer lithography (SPBCL) to obtain a diversity of nanoparticles. In SPBCL, a block copolymer is delivered onto the substrate together with the nanoparticle precursors. The block copolymer acts both as a delivery matrix to facilitate ink transport and as a confined nanoreactor that templates the growth of the nanoparticles induced by plasma reduction.161 This method was successfully employed to obtain metallic nanoparticles such as Au, Ag or Pd; metal oxide particles like Fe2O3 or Co2O3, and metal alloys of Au and Ag (Fig. 8);162 in all cases a precise control over the size and position of the particles was achieved. The same technique was used as an additional methodology to obtain CdS quantum dots by exposing the patterned Cd2+ precursor to H2S vapours.163 Using this methodology, the authors were even able to monitor the growth of the nanoparticles and study the influence of temperature and concentration of the gold precursor on the coarsening process of the particles using in situ transmission electron microscopy (TEM) experiments.164 Recently, this technique has been extended to the synthesis of multimetallic nanoparticles made from different combinations of metals (Au, Ag, Pd, Ni, Co and Pt) with precise control over their composition and shape, including the formation of Janus nanoparticles composed of immiscible metals.165 In addition, this approach was used to obtain Co3O4 nanocluster arrays that further catalyzed the localized growth of carbon nanotubes.166 PPL has also been used to pattern nanoparticle precursors using ethylene glycol as a matrix carrier in order to obtain arrays of single metallic and metal oxide nanoparticle features over extended areas.167
 | ||
| Fig. 8 HR-TEM images of different inorganic nanoparticles obtained in confined environments fabricated by SPBCL (scale bars are 2 nm). Reproduced with permission from ref. 162. | ||
On the other hand, less attention has been paid to the synthesis of purely organic nanomaterials. Our group recently reported the fabrication of the bioinspired polymer polydopamine (PDA) inside femtolitre droplets. For that, a basic solution of dopamine was coated on an AFM tip and immediately delivered on a Si/SiO2 surface, fabricating femtolitre-sized droplets that acted as confined nanoreactors where the polymerization took place. Rounded PDA features as small as 500 nm in diameter were obtained using this method (Fig. 9a). Also, local adhesion measurements and the formation of Ag nanoparticles on the in situ synthesized PDA proved that the structured material retained the adhesive and chemical properties of continuous PDA coatings, thereby confirming the viability of our approach.168
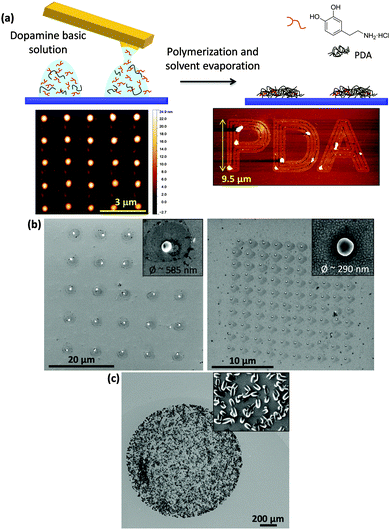 | ||
| Fig. 9 (a) Schematic representation of the experimental procedure followed to structure PDA on surfaces. A freshly prepared basic solution of dopamine was delivered onto the surface using AFM-assisted lithography in the shape of femtolitre-sized droplets where the polymerization took place. Two examples of lithographic patterns obtained with PDA are also shown, a dot-like feature array and microscale letters forming the word PDA. Adapted from ref. 168. (b) Single-particle CPP arrays obtained by delivering a mixture of reagents onto the surface and placing the substrate in a DMSO-saturated atmosphere (left) or keeping it in an oven at 50 °C (right). Adapted from ref. 169. (c) Co-bipy crystalline structures grown after deposition of droplets containing a mixture of the metal ion and the di-topic ligand. Inset: Details of the structures grown inside the droplets (unpublished results). | ||
More recently, we have reported the synthesis of coordination polymer particles (CPPs) inside droplets deposited on a surface.169 For this, we forced the reagents (an aqueous metal salt solution and an organic ligand solution) to mix on the cantilevers during the functionalization of the tips, in order to deliver a just-mixed reacting solution on the target surface. After fabricating dot-like feature arrays of the mixture, the patterned substrates were carefully stored under a highly DMSO saturated atmosphere or high temperature conditions to achieve the growth of a single particle per deposited droplet (Fig. 9b). A similar procedure was used to carry out the miniaturized synthesis of the well-known coordination polymer [Co(COOCH3)2(μ-4,4′-bipy)] (Co-bipy). Crystalline structures of Co-bipy were obtained in bulk, inside microliter-sized droplets obtained by drop-casting of their soluble precursors and confined into femtolitre droplets delivered onto an Au surface using an AFM tip (Fig. 9c). The obtained structures showed different morphologies, corresponding to the crystal growth stage reached in each case (unpublished results).
Approach III: reactions in femtolitre droplets by sequential addition of reagents
The last approach reviewed here consists of mixing solutions containing separate reagents by successively placing femtoliter sized droplets of each solution on the same location of the surface. Due to the high complexity and level of precision required, this is by far the least extended methodology of those exposed here. In fact, at the moment of elaboration of this manuscript only two articles reported this procedure in the literature.The first example was released in 2013 by Maspoch et al. and it describes the use of MPL to mix femtolitre droplets of reagents. The authors reported an extensive and very complete study that included in situ acid–base reactions detected by fluorescence microscopy and MOF synthesis and crystallization, including multiplexed arrays of Prussian blue analogs synthesized by mixing their precursors in situ.170
More recently, our group described the use of AFM-assisted lithography to synthesize CPP1 particles on mixed droplets fabricated on surfaces169 (the synthesis of the same particles by patterning a mixture of the reagents is described in the previous section). Mixed droplets were obtained by delivering each one of the reagent solutions separately on the same position of the surface using a multiple cantilever array. After exposing the substrate patterned with the mixed droplets to a DMSO atmosphere, the growth of a single particle inside each droplet was achieved (Fig. 10a). This procedure also allowed to observe the formation of primitive dendritic structures and their evolution to the final particles (Fig. 10b).169
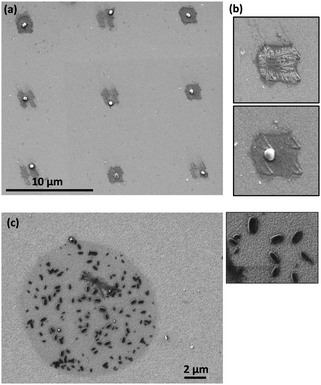 | ||
| Fig. 10 (a) Single CPPs grown inside mixed droplets obtained by depositing each of the inks on the same position of the surface. After patterning, the substrate was kept in a DMSO-saturated atmosphere for 48 hours and under atmospheric conditions for 48 more hours. (b) Details of one of the structures in the array. Top: After the first 48 hours a dendritic structure was observed. Bottom: Two days later, the dendritic structure had evolved to form a single rounded particle. Adapted with permission from ref. 169. (c) Co-bipy crystals obtained after sequential delivery of the reagents and confined reaction and crystallization. Inset: Details of the structures grown inside the droplets (unpublished results). | ||
Similarly, the sequential delivery of reagents onto a surface in the shape of ultra-small droplets was used to synthesize Co-bipy nanocrystals directly on the surface. The coordination reaction between the metal centres and the ditopic ligands was also carried out in femtolitre-sized droplets fabricated on a gold surface and crystalline structures were obtained (Fig. 10c). Micro Raman spectroscopy was used to characterize the materials and confirmed the formation of the Co-bipy coordination polymer.
Conclusions
As summarized here, the use of tip-assisted methodologies to perform confined chemical reactions in femtolitre droplets has gained relevance over the last years. To summarize the different examples so far reported, we have grouped them into three main categories. The first and most widely used category groups the different reactions induced upon contact of the delivered droplets with the substrate. Experimentally, this is the simplest approach out of the three and has been used to perform a variety of chemical reactions including metal ion reduction and click chemistry.In the second approach, different reactants are already contained in the solution and react (either spontaneously or induced by an external stimulus) upon deposition of the femtolitre droplets on the surface. The examples found in the literature concerning this methodology are mostly focused on the on-surface synthesis of inorganic nanomaterials, with only a few examples reporting the synthesis of metal–organic systems and purely organic materials.
The third and final approach is definitely the most unusual out of the three, mainly because of the difficulty that implies placing two ultra-small droplets in the same position on a surface with nanometric X–Y resolution while ensuring their effective mixing (Z). However, as the reagents are mixed directly on the surface, it is the only methodology that allows patterning of nanomaterials with fast growth reaction kinetics, thereby opening new application venues.
Overall, several successful examples of tip-assisted chemistry within femtolitre droplets have already been described. Nevertheless, this technology can still be considered to be in the early stages of development. For this reason, and to extend the applicability of this approach, several challenges still remain to be faced in the near future. First of all, the reproducibility of the experiments (especially in the third approach) should increase. Also, a wider range of reactions should be performed in this way; whilst many biochemical processes have been studied in microemulsions and microfluidic-generated droplets, these are not yet represented in femtolitre tip-assisted chemistry. Similar considerations are valid for purely organic chemical reactions. However, for that to happen, as important as the synthesis itself is the development of suitable experimental techniques to characterize the materials beyond the imaging-based techniques that have been mostly used until now. This is probably the most important and difficult challenge that we are facing at the moment, and overcoming it will only be possible through the collaboration between scientists of multiple disciplines.
Acknowledgements
M. G. thanks the CSIC for a predoctoral grant (JAEpre). This work was supported by projects MAT2015-70615-R and CTQ2013-41161-R from the Spanish Government and by FEDER funds. ICN2 acknowledges support from the Severo Ochoa Program (MINECO, Grant SEV-2013-0295).Notes and references
- D. T. Chiu, R. M. Lorenz and G. D. M. Jeffries, Anal. Chem., 2009, 81, 5111–5118 CrossRef CAS PubMed.
- H. H. Gorris and D. R. Walt, Angew. Chem., Int. Ed., 2010, 49, 3880–3895 CrossRef CAS PubMed.
- C. P. Collier and M. L. Simpson, Curr. Opin. Biotechnol., 2011, 22, 516–526 CrossRef CAS PubMed.
- A. Fallah-Araghi, K. Meguellati, J. C. Baret, A. El Harrak, T. Mangeat, M. Karplus, S. Ladame, C. M. Marques and A. D. Griffiths, Phys. Rev. Lett., 2014, 112, 28301–28305 CrossRef PubMed.
- A. K. Ganguli, A. Ganguly and S. Vaidya, Chem. Soc. Rev., 2010, 39, 474–485 RSC.
- A. B. Theberge, F. Courtois, Y. Schaerli, M. Fischlechner, C. Abell, F. Hollfelder and W. T. S. Huck, Angew. Chem., Int. Ed., 2010, 49, 5846–5868 CrossRef CAS PubMed.
- S. A. Bode, I. J. Minten, R. J. M. Nolte and J. J. L. M. Cornelissen, Nanoscale, 2011, 3, 2376–2389 RSC.
- J. Bibette, F. Leal Calderon and P. Poulin, Rep. Prog. Phys., 1999, 62, 969–1033 CrossRef CAS.
- M. Nakano, J. Komatsu, S. I. Matsuura, K. Takashima, S. Katsura and A. Mizuno, J. Biotechnol., 2003, 102, 117–124 CrossRef CAS PubMed.
- A. V. Pietrini and P. L. Luisi, ChemBioChem, 2004, 5, 1055–1062 CrossRef CAS PubMed.
- V. Noireaux and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17669–17674 CrossRef CAS PubMed.
- D. Stamou, C. Duschl, E. Delamarche and H. Vogel, Angew. Chem., Int. Ed., 2003, 42, 5580–5583 CrossRef CAS PubMed.
- D. T. Chiu, C. F. Wilson, F. Ryttsén, A. Strömberg, C. Farre, A. Karlsson, S. Nordholm, A. Gaggar, B. P. Modi, A. Moscho, R. A. Garza-López, O. Orwar and R. N. Zare, Science, 1999, 283, 1892–1895 CrossRef CAS PubMed.
- P. Y. Bolinger, D. Stamou and H. Vogel, Angew. Chem., Int. Ed., 2008, 47, 5544–5549 CrossRef CAS PubMed.
- L. Hosta-Rigau, M. J. York-Duran, Y. Zhang, K. N. Goldie and B. Städler, ACS Appl. Mater. Interfaces, 2014, 6, 12771–12779 CAS.
- C. Aubery, C. Solans, S. Prevost, M. Gradzielski and M. Sanchez-Dominguez, Langmuir, 2013, 29, 1779–1789 CrossRef CAS PubMed.
- L. M. Magno, D. G. Angelescu, W. Sigle and C. Stubenrauch, Phys. Chem. Chem. Phys., 2011, 13, 3048–3058 RSC.
- F. Heshmatpour and R. Abazari, RSC Adv., 2014, 4, 55815–55826 RSC.
- I. Capek, Adv. Colloid Interface Sci., 2004, 110, 49–74 CrossRef CAS PubMed.
- C.-H. Lin, J.-H. Chang, Y.-Q. Yeh, S.-H. Wu, Y.-H. Liu and C.-Y. Mou, Nanoscale, 2015, 7, 9614–9626 RSC.
- D. Qi, Z. Cao and U. Ziener, Adv. Colloid Interface Sci., 2014, 211, 47–62 CrossRef CAS PubMed.
- A. Carne, C. Carbonell, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2011, 40, 291–305 RSC.
- M. Sanchez-Dominguez, K. Pemartin and M. Boutonnet, Curr. Opin. Colloid Interface Sci., 2012, 17, 297–305 CrossRef CAS.
- S. Vaucher, M. Li and S. Mann, Angew. Chem., Int. Ed., 2000, 39, 1793–1796 CrossRef CAS.
- M. F. Dumont, O. N. Risset, E. S. Knowles, T. Yamamoto, D. M. Pajerowski, M. W. Meisel and D. R. Talham, Inorg. Chem., 2013, 52, 4494–4501 CrossRef CAS PubMed.
- Y. Liu and X. Wang, Polym. Chem., 2012, 3, 2632–2639 RSC.
- X. Roy, J. K. H. Hui, M. Rabnawaz, G. Liu and M. J. MacLachlan, J. Am. Chem. Soc., 2011, 133, 8420–8423 CrossRef CAS PubMed.
- S. Ye, Y. Liu, S. Chen, S. Liang, R. McHale, N. Ghasdian, Y. Lu and X. Wang, Chem. Commun., 2011, 47, 6831–6833 RSC.
- W. J. Rieter, K. M. L. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2006, 128, 9024–9025 CrossRef CAS PubMed.
- W. Lin, W. J. Rieter and K. M. L. Taylor, Angew. Chem., Int. Ed., 2009, 48, 650–658 CrossRef CAS PubMed.
- K. M. L. Taylor, W. J. Rieter and W. Lin, J. Am. Chem. Soc., 2008, 130, 14358–14359 CrossRef CAS PubMed.
- D. Tanaka, A. Henke, K. Albrecht, M. Moeller, K. Nakagawa, S. Kitagawa and J. Groll, Nat. Chem., 2010, 2, 410–416 CrossRef CAS PubMed.
- A. Tokarev, L. Salmon, Y. Guari, G. Molnár and A. Bousseksou, New J. Chem., 2011, 35, 2081–2088 RSC.
- I. Boldog, A. B. Gaspar, V. Martínez, P. Pardo-Ibañez, V. Ksenofontov, A. Bhattacharjee, P. Gütlich and J. A. Real, Angew. Chem., Int. Ed., 2008, 47, 6433–6437 CrossRef CAS PubMed.
- T. Forestier, S. Mornet, N. Daro, T. Nishihara, S. Mouri, K. Tanaka, O. Fouché, E. Freysz and J.-F. Létard, Chem. Commun., 2008, 4327–4329 RSC.
- F. Volatron, L. Catala, E. Rivière, A. Gloter, O. Stéphan and T. Mallah, Inorg. Chem., 2008, 47, 6584–6586 CrossRef CAS PubMed.
- A. E. C. Palmqvist, Curr. Opin. Colloid Interface Sci., 2003, 8, 164–178 CrossRef.
- K. Ouadahi, E. Allard, B. Oberleitner and C. Larpent, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 314–328 CrossRef CAS.
- V. Monteil, P. Wehrmann and S. Mecking, J. Am. Chem. Soc., 2005, 127, 14568–14569 CrossRef CAS PubMed.
- K. Landfester, M. Willert and M. Antonietti, Macromolecules, 2000, 33, 2370–2376 CrossRef CAS.
- W. Zhang and Q. Zhong, J. Agric. Food Chem., 2009, 57, 9181–9189 CrossRef CAS PubMed.
- D. Sağlam, P. Venema, E. van der Linden and R. de Vries, Curr. Opin. Colloid Interface Sci., 2014, 19, 428–437 CrossRef.
- A. de la Escosura, R. J. M. Nolte and J. J. L. M. Cornelissen, J. Mater. Chem., 2009, 19, 2274 RSC.
- K. T. Kim, S. A. Meeuwissen, R. J. M. Nolte and J. C. M. van Hest, Nanoscale, 2010, 2, 844–858 RSC.
- L. Schoonen and J. C. M. van Hest, Nanoscale, 2014, 6, 7124–7141 RSC.
- S. Abe, B. Maity and T. Ueno, Chem. Commun., 2016, 52, 6496–6512 RSC.
- A. A. A. Aljabali, F. Sainsbury, G. P. Lomonossoff and D. J. Evans, Small, 2010, 6, 818–821 CrossRef CAS PubMed.
- T. Ueno, M. Suzuki, T. Goto, T. Matsumoto, K. Nagayama and Y. Watanabe, Angew. Chem., Int. Ed., 2004, 43, 2527–2530 CrossRef CAS PubMed.
- B. Maity, K. Fujita and T. Ueno, Curr. Opin. Chem. Biol., 2015, 25, 88–97 CrossRef CAS PubMed.
- J. E. Glasgow, M. A. Asensio, C. M. Jakobson, M. B. Francis and D. Tullman-Ercek, ACS Synth. Biol., 2015, 4, 1011–1019 CrossRef CAS PubMed.
- D. P. Patterson, B. Schwarz, R. S. Waters, T. Gedeon and T. Douglas, ACS Chem. Biol., 2014, 9, 359–365 CrossRef CAS PubMed.
- M. G. Ryadnov, Angew. Chem., Int. Ed., 2007, 46, 969–972 CrossRef CAS PubMed.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336–7356 CrossRef CAS PubMed.
- B. Zheng and R. F. Ismagilov, Angew. Chem., Int. Ed., 2005, 44, 2520–2523 CrossRef CAS PubMed.
- B. Zheng, J. D. Tice, L. S. Roach and R. F. Ismagilov, Angew. Chem., Int. Ed., 2004, 43, 2508–2511 CrossRef CAS PubMed.
- T. Panagiotou, S. V. Mesite and R. J. Fisher, Ind. Eng. Chem. Res., 2009, 48, 1761–1771 CrossRef CAS.
- J. M. Köhler, T. Henkel, A. Grodrian, T. Kirner, M. Roth, K. Martin and J. Metze, Chem. Eng. J., 2004, 101, 201–216 CrossRef.
- T. Hatakeyama, D. L. Chen and R. F. Ismagilov, J. Am. Chem. Soc., 2006, 128, 2518–2519 CrossRef CAS PubMed.
- Y. Önal, M. Lucas and P. Claus, Chem. Eng. Technol., 2005, 28, 972–978 CrossRef.
- A. Liau, R. Kamik, A. Majumdar and J. H. D. Cate, Anal. Chem., 2005, 77, 7618–7625 CrossRef CAS PubMed.
- A. Aharoni, A. D. Griffiths and D. S. Tawfik, Curr. Opin. Chem. Biol., 2005, 9, 210–216 CrossRef CAS PubMed.
- P. S. Dittrich, M. Jahnz and P. Schwille, ChemBioChem, 2005, 6, 811–814 CrossRef CAS PubMed.
- K. Martin, T. Henkel, V. Baier, A. Grodrian, T. Schön, M. Roth, J. Michael Köhler and J. Metze, Lab Chip, 2003, 3, 202–207 RSC.
- A. Grodrian, J. Metze, T. Henkel, K. Martin, M. Roth and J. M. Köhler, Biosens. Bioelectron., 2004, 19, 1421–1428 CrossRef CAS PubMed.
- G. Niu, A. Ruditskiy, M. Vara and Y. Xia, Chem. Soc. Rev., 2015, 44, 5806–5820 RSC.
- A. M. Nightingale and J. C. DeMello, Adv. Mater., 2013, 25, 1813–1821 CrossRef CAS PubMed.
- S. Marre and K. F. Jensen, Chem. Soc. Rev., 2010, 39, 1183–1202 RSC.
- D. Shalom, R. C. R. Wootton, R. F. Winkle, B. F. Cottam, R. Vilar, A. J. DeMello and C. P. Wilde, Mater. Lett., 2007, 61, 1146–1150 CrossRef CAS.
- S. Duraiswamy and S. A. Khan, Small, 2009, 5, 2828–2834 CrossRef CAS PubMed.
- B. K. H. Yen, A. Günther, M. A. Schmidt, K. F. Jensen and M. G. Bawendi, Angew. Chem., Int. Ed., 2005, 44, 5447–5451 CrossRef CAS PubMed.
- K. Kumar, A. M. Nightingale, S. H. Krishnadasan, N. Kamaly, M. Wylenzinska-Arridge, K. Zeissler, W. R. Branford, E. Ware, A. J. DeMello and J. C. DeMello, J. Mater. Chem., 2012, 22, 4704–4708 RSC.
- K. I. Sotowa, K. Irie, T. Fukumori, K. Kusakabe and S. Sugiyama, Chem. Eng. Technol., 2007, 30, 383–388 CrossRef CAS.
- P. H. Hoang, H. Park and D. P. Kim, J. Am. Chem. Soc., 2011, 133, 14765–14770 CrossRef CAS PubMed.
- S. Duraiswamy and S. A. Khan, Nano Lett., 2010, 10, 3757–3763 CrossRef CAS PubMed.
- S. Takeuchi, P. Garstecki, D. B. Weibel and G. M. Whitesides, Adv. Mater., 2005, 17, 1067–1072 CrossRef CAS.
- S. Xu, Z. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin and G. M. Whitesides, Angew. Chem., Int. Ed., 2005, 44, 724–728 CrossRef CAS PubMed.
- M. Faustini, J. Kim, G. Y. Jeong, J. Y. Kim, H. R. Moon, W. S. Ahn and D. P. Kim, J. Am. Chem. Soc., 2013, 135, 14619–14626 CrossRef CAS PubMed.
- L. Paseta, B. Seoane, D. Julve, V. Sebastián, C. Téllez and J. Coronas, ACS Appl. Mater. Interfaces, 2013, 5, 9405–9410 CAS.
- M. P. Batten, M. Rubio-Martinez, T. Hadley, K. Carey, K. L. A. Polyzos and M. R. Hill, Curr. Opin. Chem. Eng., 2015, 8, 55–59 CrossRef.
- P. Pantano and D. R. Walt, Chem. Mater., 1996, 8, 2832–2835 CrossRef CAS.
- D. R. Walt, Chem. Soc. Rev., 2010, 39, 38–50 RSC.
- Y. Men, Y. Fu, Z. Chen, P. A. Sims, W. J. Greenleaf and Y. Huang, Anal. Chem., 2012, 84, 4262–4266 CrossRef CAS PubMed.
- M. J. Levene, J. Korlach, S. W. Turner, M. Foquet, H. G. Craighead and W. W. Webb, Science, 2003, 299, 682–686 CrossRef CAS PubMed.
- R. Garcia, A. W. Knoll and E. Riedo, Nat. Nanotechnol., 2014, 9, 577–587 CrossRef CAS PubMed.
- M. Jaschke and H.-J. Butt, Langmuir, 1995, 11, 1061–1064 CrossRef CAS.
- R. D. Piner, J. Zhu, F. Xu, S. Hong and C. A. Mirkin, Science, 1999, 283, 661–663 CrossRef CAS PubMed.
- S. Hong, J. Zhu and C. A. Mirkin, Langmuir, 1999, 15, 7897–7900 CrossRef CAS.
- S. Hong, J. Zhu and C. A. Mirkin, Science, 1999, 286, 523–525 CrossRef CAS PubMed.
- S. Hong and C. A. Mirkin, Science, 2000, 288, 1808–1811 CrossRef CAS PubMed.
- B. Basnar and I. Willner, Small, 2009, 5, 28–44 CrossRef CAS PubMed.
- D. S. Ginger, H. Zhang and C. A. Mirkin, Angew. Chem., Int. Ed., 2004, 43, 30–45 CrossRef PubMed.
- R. J. Barsotti, M. S. O'Connell and F. Stellacci, Langmuir, 2004, 20, 4795–4798 CrossRef CAS PubMed.
- H. Zhang, S. Chung and C. A. Mirkin, Nano Lett., 2003, 3, 43–45 CrossRef CAS.
- C.-C. Wu, D. N. Reinhoudt, C. Otto, V. Subramaniam and A. H. Velders, Small, 2011, 7, 989–1002 CrossRef CAS PubMed.
- F. Brinkmann, M. Hirtz, A. M. Greiner, M. Weschenfelder, B. Waterkotte, M. Bastmeyer and H. Fuchs, Small, 2013, 9, 3266–3275 CrossRef CAS PubMed.
- E. J. Irvine, A. Hernandez-Santana, K. Faulds and D. Graham, Analyst, 2011, 136, 2925–2930 RSC.
- Z. Xie, X. Zhou, X. Tao and Z. Zheng, Macromol. Rapid Commun., 2012, 33, 359–373 CrossRef CAS PubMed.
- Y. H. Shin, S. H. Yun, S. H. Pyo, Y. S. Lim, H. J. Yoon, K. H. Kim, S. K. Moon, S. W. Lee, Y. G. Park, S. I. Chang, K. M. Kim and J. H. Lim, Angew. Chem., Int. Ed., 2010, 49, 9689–9692 CrossRef CAS PubMed.
- M. A. Kramer, R. L. Gieseck, B. Andrews and A. Ivanisevic, J. Am. Chem. Soc., 2011, 133, 9627–9629 CrossRef CAS PubMed.
- L. M. Demers, D. S. Ginger, S.-J. Park, Z. Li, S.-W. Chung and C. A. Mirkin, Science, 2002, 296, 1836–1838 CrossRef CAS PubMed.
- C. O. Connell, M. Higgins, S. Moulton and G. Wallace, J. Mater. Chem. C, 2015, 3, 6431–6444 RSC.
- J. Zhong, G. Sun and D. He, Nanoscale, 2014, 6, 12217–12228 RSC.
- L. Petersson, L. Dexlin-Mellby, A. A. Bengtsson, G. Sturfelt, C. A. K. Borrebaeck and C. Wingren, Lab Chip, 2014, 14, 1931–1942 RSC.
- X. Zhou, S. He, K. A. Brown, J. Mendez-Arroyo, F. Boey and C. A. Mirkin, Nano Lett., 2013, 13, 1616–1621 CAS.
- F. Huo, Z. Zheng, G. Zheng, L. R. Giam, H. Zhang and C. A. Mirkin, Science, 2008, 321, 1658–1660 CrossRef CAS PubMed.
- Z. Xie, C. Chen, X. Zhou, T. Gao, D. Liu, Q. Miao and Z. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 11955–11964 CAS.
- Z. Mao, M. Ganesh, M. Bucaro, I. Smolianski, R. A. Gross and A. M. Lyons, Biomacromolecules, 2014, 15, 4627–4636 CrossRef CAS PubMed.
- L. R. Giam, M. D. Massich, L. Hao, L. Shin Wong, C. C. Mader and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4377–4382 CrossRef CAS PubMed.
- Z. Zheng, W. L. Daniel, L. R. Giam, F. Huo, A. J. Senesi, G. Zheng and C. A. Mirkin, Angew. Chem., Int. Ed., 2009, 48, 7626–7629 CrossRef CAS PubMed.
- L. R. Giam and C. A. Mirkin, Angew. Chem., Int. Ed., 2011, 50, 7482–7485 CrossRef CAS PubMed.
- L. Huang, A. B. Braunschweig, W. Shim, L. Qin, J. K. Lim, S. J. Hurst, F. Huo, C. Xue, J. W. Jang and C. A. Mirkin, Small, 2010, 6, 1077–1081 CrossRef CAS PubMed.
- D. J. Eichelsdoerfer, K. A. Brown, R. Boya, W. Shim and C. A. Mirkin, Nano Lett., 2013, 13, 664–667 CrossRef CAS PubMed.
- D. L. Wilson, R. Martin, S. Hong, M. Cronin-Golomb, C. A. Mirkin and D. L. Kaplan, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 13660–13664 CrossRef CAS PubMed.
- A. J. Senesi, D. I. Rozkiewicz, D. N. Reinhoudt and C. A. Mirkin, ACS Nano, 2009, 3, 2394–2402 CrossRef CAS PubMed.
- G. Agarwal, R. R. Naik and M. O. Stone, J. Am. Chem. Soc., 2003, 125, 7408–7412 CrossRef CAS PubMed.
- J. Chai, L. S. Wong, L. Giam and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 19521–19525 CrossRef CAS PubMed.
- K.-B. Lee, S.-J. Park, C. A. Mirkin, J. C. Smith and M. Mrksich, Science, 2002, 295, 1702–1705 CrossRef CAS PubMed.
- G. Arrabito, S. Reisewitz, L. Dehmelt, P. I. Bastiaens, B. Pignataro, H. Schroeder and C. M. Niemeyer, Small, 2013, 9, 4243–4249 CrossRef CAS PubMed.
- J. Kim, Y. H. Shin, S. H. Yun, D. S. Choi, J. H. Nam, S. R. Kim, S. K. Moon, B. H. Chung, J. H. Lee, J. H. Kim, K. Y. Kim, K. M. Kim and J. H. Lim, J. Am. Chem. Soc., 2012, 134, 16500–16503 CrossRef CAS PubMed.
- H. Nakashima, M. J. Higgins, C. O'Connell, K. Torimitsu and G. G. Wallace, Langmuir, 2012, 28, 804–811 CrossRef PubMed.
- H. Li, X. Cao, B. Li, X. Zhou, G. Lu, C. Liusman, Q. He, F. Boey, S. S. Venkatraman and H. Zhang, Chem. Commun., 2011, 47, 10070–10072 RSC.
- M. Hirtz, A. Oikonomou, T. Georgiou, H. Fuchs and A. Vijayaraghavan, Nat. Commun., 2013, 4, 2591 Search PubMed.
- E. Bellido, I. Ojea-Jiménez, A. Ghirri, C. Alvino, A. Candini, V. Puntes, M. Affronte, N. Domingo and D. Ruiz-Molina, Langmuir, 2012, 28, 12400–12409 CrossRef CAS PubMed.
- R. Sistiabudi and A. Ivanisevic, Adv. Mater., 2008, 20, 3678–3681 CrossRef CAS.
- M. A. Kramer, H. C. Park and A. Ivanisevic, Scanning, 2010, 32, 30–34 CAS.
- M. J. Martínez-Pérez, E. Bellido, R. De Miguel, J. Sesé, A. Lostao, C. Gómez-Moreno, D. Drung, T. Schurig, D. Ruiz-Molina and F. Luis, Appl. Phys. Lett., 2011, 99, 10–13 CrossRef.
- E. Bellido, P. González-Monje, A. Repollés, M. Jenkins, J. Sesé, D. Drung, T. Schurig, K. Awaga, F. Luis and D. Ruiz-Molina, Nanoscale, 2013, 5, 12565–12573 RSC.
- P. Manandhar, K.-S. Chen, K. Aledealat, G. Mihajlović, C. S. Yun, M. Field, G. J. Sullivan, G. F. Strouse, P. B. Chase, S. von Molnár and P. Xiong, Nanotechnology, 2009, 20, 355501 CrossRef PubMed.
- B. W. Maynor, J. Li, C. Lu and J. Liu, J. Am. Chem. Soc., 2004, 126, 6409–6413 CrossRef CAS PubMed.
- S. Rozhok, R. Piner and C. A. Mirkin, J. Phys. Chem. B, 2003, 107, 751–757 CrossRef CAS.
- S. Rozhok, P. Sun, R. Piner, M. Lieberman and C. A. Mirkin, J. Phys. Chem. B, 2004, 108, 7814–7819 CrossRef CAS.
- W. M. Wang, R. M. Stoltenberg, S. Liu and Z. Bao, ACS Nano, 2008, 2, 2135–2142 CrossRef CAS PubMed.
- H. Jung, C. K. Dalal, S. Kuntz, R. Shah and C. P. Collier, Nano Lett., 2004, 4, 2171–2177 CrossRef CAS.
- C. D. O'Connell, M. J. Higgins, R. P. Sullivan, S. E. Moulton and G. G. Wallace, Small, 2014, 10, 3717–3728 CrossRef PubMed.
- K. A. Brown, D. J. Eichelsdoerfer, X. Liao, S. He and C. A. Mirkin, Front. Phys., 2014, 9, 385–397 CrossRef.
- B. W. Maynor, Y. Li and J. Liu, Langmuir, 2001, 17, 2575–2578 CrossRef CAS.
- L. A. Porter, H. C. Choi, J. M. Schmeltzer, A. E. Ribbe, L. C. C. Elliott and J. M. Buriak, Nano Lett., 2002, 2, 1369–1372 CrossRef CAS.
- H. Chu, Z. Jin, Y. Zhang, W. Zhou, L. Ding and Y. Li, J. Phys. Chem. C, 2008, 112, 13437–13441 CAS.
- D. A. Long, K. Unal, R. C. Pratt, M. Malkoch and J. Frommer, Adv. Mater., 2007, 19, 4471–4473 CrossRef CAS.
- H. Y. Chen, M. Hirtz, X. Deng, T. Laue, H. Fuchs and J. Lahann, J. Am. Chem. Soc., 2010, 132, 18023–18025 CrossRef CAS PubMed.
- S. Oberhansl, M. Hirtz, A. Lagunas, R. Eritja, E. Martinez, H. Fuchs and J. Samitier, Small, 2012, 8, 541–545 CrossRef CAS PubMed.
- A. Martínez-Otero, P. González-Monje, D. Maspoch, J. Hernando and D. Ruiz-Molina, Chem. Commun., 2011, 47, 6864–6866 RSC.
- S. Bian, J. He, K. B. Schesing and A. B. Braunschweig, Small, 2012, 8, 2000–2005 CrossRef CAS PubMed.
- S. Bian, K. B. Schesing and A. B. Braunschweig, Chem. Commun., 2012, 48, 4995–4997 RSC.
- S. Bian, A. M. Scott, Y. Cao, Y. Liang, S. Osuna, K. N. Houk and A. B. Braunschweig, J. Am. Chem. Soc., 2013, 135, 9240–9243 CrossRef CAS PubMed.
- X. Han, S. Bian, Y. Liang, K. N. Houk and A. B. Braunschweig, J. Am. Chem. Soc., 2014, 136, 10553–10556 CrossRef CAS PubMed.
- F. Huo, G. Zheng, X. Liao, L. R. Giam, J. Chai, X. Chen, W. Shim and C. A. Mirkin, Nat. Nanotechnol., 2010, 5, 637–640 CrossRef CAS PubMed.
- S. Bian, S. B. Zieba, W. Morris, X. Han, D. C. Richter, K. A. Brown, C. A. Mirkin and A. B. Braunschweig, Chem. Sci., 2014, 5, 2023 RSC.
- E. Bellido, S. Cardona-Serra, E. Coronado and D. Ruiz-Molina, Chem. Commun., 2011, 47, 5175–5177 RSC.
- C. Carbonell, I. Imaz and D. Maspoch, J. Am. Chem. Soc., 2011, 133, 2144–2147 CrossRef CAS PubMed.
- J. Xu, M. Lynch, J. L. Huff, C. Mosher, S. Vengasandra, G. Ding and E. Henderson, Biomed. Microdevices, 2004, 6, 117–123 CrossRef CAS PubMed.
- M. Su, X. Liu, S. Y. Li, V. P. Dravid and C. A. Mirkin, J. Am. Chem. Soc., 2002, 124, 1560–1561 CrossRef CAS PubMed.
- L. Ding, Y. Li, H. Chu, X. Li and J. Liu, J. Phys. Chem. B, 2005, 109, 22337–22340 CrossRef CAS PubMed.
- H. Chu, L. Ding, J. Wang, X. Li, L. You and Y. Li, J. Phys. Chem. C, 2008, 112, 18938–18942 CAS.
- L. Fu, X. Liu, Y. Zhang, V. P. Dravid and C. A. Mirkin, Nano Lett., 2003, 3, 757–760 CrossRef CAS.
- A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18–52 CrossRef CAS PubMed.
- S. Kinge, M. Crego-Calama and D. N. Reinhoudt, ChemPhysChem, 2008, 9, 20–42 CrossRef CAS PubMed.
- J. C. Garno, Y. Yang, N. A. Amro, S. Cruchon-Dupeyrat, S. Chen and G. Y. Liu, Nano Lett., 2003, 3, 389–395 CrossRef CAS.
- T. Danieli and D. Mandler, J. Solid State Electrochem., 2013, 17, 2989–2997 CrossRef CAS.
- R. J. Barsotti Jr. and F. Stellacci, J. Mater. Chem., 2006, 16, 962–965 RSC.
- J. Chai, F. Huo, Z. Zheng, L. R. Giam, W. Shim and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20202–20206 CrossRef CAS PubMed.
- G. Liu, D. J. Eichelsdoerfer, B. Rasin, Y. Zhou, K. A. Brown, X. Liao and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 887–891 CrossRef CAS PubMed.
- L. R. Giam, S. He, N. E. Horwitz, D. J. Eichelsdoerfer, J. Chai, Z. Zheng, D. Kim, W. Shim and C. A. Mirkin, Nano Lett., 2012, 12, 1022–1025 CrossRef CAS PubMed.
- J. Chai, X. Liao, L. R. Giam and C. A. Mirkin, J. Am. Chem. Soc., 2012, 134, 158–161 CrossRef CAS PubMed.
- P. C. Chen, G. Liu, Y. Zhou, K. A. Brown, N. Chernyak, J. L. Hedrick, S. He, Z. Xie, Q.-Y. Lin, V. P. Dravid, S. A. O'Neill-Slawecki and C. A. Mirkin, J. Am. Chem. Soc., 2015, 137, 9167–9173 CrossRef CAS PubMed.
- A. B. Smetana, S. Pacley, J. Boeckl, P. Adamczyk and S. Nettikadan, J. Mater. Chem. C, 2013, 1, 1798–1803 RSC.
- J. Wu, X. Zan, S. Li, Y. Liu, C. Cui, B. Zou, W. Zhang, H. Xu, H. Duan, D. Tian, W. Huang and F. Huo, Nanoscale, 2013, 6, 749–752 RSC.
- M. Guardingo, M. J. Esplandiu and D. Ruiz-Molina, Chem. Commun., 2014, 50, 12548–12551 RSC.
- M. Guardingo, P. Gonzalez-Monje, F. Novio, E. Bellido, F. Busque, G. Molnar, A. Bousseksou and D. Ruiz-Molina, ACS Nano, 2016, 10, 3206–3213 CrossRef CAS PubMed.
- C. Carbonell, K. C. Stylianou, J. Hernando, E. Evangelio, S. A. Barnett, S. Nettikadan, I. Imaz and D. Maspoch, Nat. Commun., 2013, 4, 2173 Search PubMed.
Footnote |
| † Present address: MDPI Spain, Av. Madrid 95, 08028 Barcelona, Spain. |
| This journal is © The Royal Society of Chemistry 2016 |