Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A divergent synthetic approach to diverse molecular scaffolds: assessment of lead-likeness using LLAMA, an open-access computational tool†
Ignacio
Colomer
a,
Christopher J.
Empson
 ab,
Philip
Craven
ab,
Philip
Craven
 ab,
Zachary
Owen
a,
Richard G.
Doveston
ab,
Ian
Churcher
c,
Stephen P.
Marsden
*a and
Adam
Nelson
*ab
ab,
Zachary
Owen
a,
Richard G.
Doveston
ab,
Ian
Churcher
c,
Stephen P.
Marsden
*a and
Adam
Nelson
*ab
aSchool of Chemistry, University of Leeds, Leeds, LS2 9JT, UK. E-mail: s.p.marsden@leeds.ac.uk; a.s.nelson@leeds.ac.uk
bAstbury Centre for Structural Molecular Biology, University of Leeds, Leeds, LS2 9JT, UK
cGlaxoSmithKline Medicines Research Centre, Stevenage, SG1 2NY, UK
First published on 5th May 2016
Abstract
Complementary cyclisation reactions of hex-2-ene-1,6-diamine derivatives were exploited in the synthesis of alternative molecular scaffolds. The value of the synthetic approach was analysed using LLAMA, an open-access computational tool for assessing the lead-likeness and novelty of molecular scaffolds.
Controlling molecular properties is crucial in drug discovery because the probability of successful progression is influenced by parameters including lipophilicity, molecular weight, the number of aromatic rings and the fraction of sp3-hybridised carbons (Fsp3).1 As a result, guidelines, such as Lipinski's rule-of-five (concerning oral bioavailability),2 have been formulated to help chemists to target drug-relevant chemical space.3
In turn, controlling the molecular properties of lead compounds is advisable since optimisation generally increases lipophilicity, molecular weight and complexity.4 As a result, lead-like chemical space can be described in terms of both molecular properties (e.g.5 −1 < c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 3; 14 ≤ heavy atoms ≤ 26) and structural features.5 Unfortunately, most commercially-available compounds5,6
P < 3; 14 ≤ heavy atoms ≤ 26) and structural features.5 Unfortunately, most commercially-available compounds5,6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡ and new synthetic methods5 do not target lead-like chemical space. The problem is exacerbated when diversity is considered since chemical space has been explored very unevenly and unsystematically.7
‡ and new synthetic methods5 do not target lead-like chemical space. The problem is exacerbated when diversity is considered since chemical space has been explored very unevenly and unsystematically.7
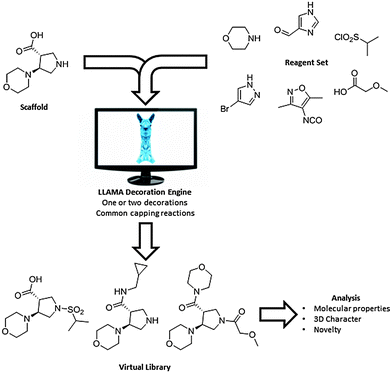
The challenge5,8 of exploring novel lead-like chemical space has prompted us6b,9 and others10 to develop lead-oriented synthetic approaches. Demonstrating the value of such approaches requires tools for virtual library enumeration and evaluation that are not commonly available within academia. We have therefore developed LLAMA (Lead-Likeness and Molecular Analysis),§ an open-access tool that enables decoration11 and assessment of the lead-likeness of small molecule scaffolds (Fig. 1). Each product is assigned a “lead-likeness penalty” (LLP) which penalises properties and features that are not lead-like (Fig. 2; ESI†). Rather than applying strict filters, the penalty increases with deviation from lead-like space. Scaffold novelty is assessed by comparing the corresponding Murcko assemblies12 (with and without alpha atoms) with those of, or embedded in, a random 2% of the “available now” set of the ZINC database13 of commercially-available compounds. Finally, to capture the shape diversity of the compounds, the principal moments of inertia14a (PMI) and mean deviation from the plane of best fit14b of low-lying conformers are determined. To demonstrate LLAMA's utility, we analysed the lead-likeness of some scaffolds prepared using an approach that we have developed.
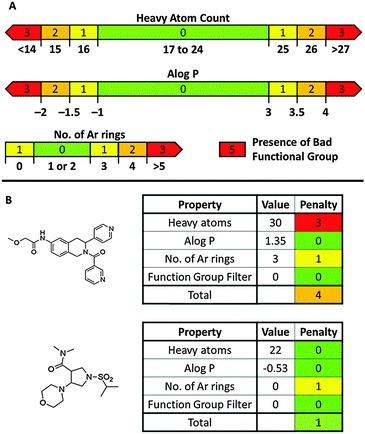 | ||
| Fig. 2 Lead-likeness penalty. Panel A: Graphical representation of contributions to the penalty. Panel B: Penalties for two exemplar compounds. | ||
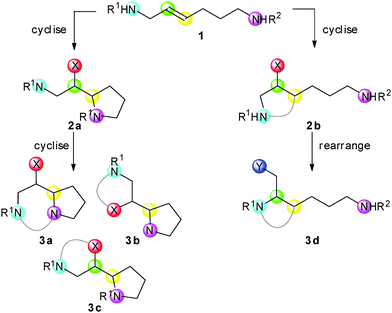
We envisaged a divergent synthetic approach in which unsymmetrical unsaturated diamine derivatives 1 would be converted into alternative scaffolds (Scheme 1). Thus, complementary cyclisations would yield alternative heterocyclic intermediates 2; further cyclisation or rearrangement would then yield additional molecular scaffolds 3. The approach would be reminiscent of a branching pathway approach which enabled a single substrate to be converted into twelve natural product-like scaffolds.15
The fate of the iodocyclisation reactions of hex-2-ene-diamine derivatives 1 was dependent on the specific protecting groups used (Scheme 2). Thus, treatment of the differentially-protected 1a with iodine and sodium bicarbonate in acetonitrile resulted in cyclisation of the remote Boc-protected amine to yield the pyrrolidine 4 in 76% yield.16 In stark contrast, under the same conditions, the doubly Boc-protected amine 1b cyclised through the allylic carbamate to give a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of the oxazinone 7 and the corresponding oxazolidinone (66% and 4% yield respectively).17 In each case, regioselectivity was determined using the diagnostic upfield 13C NMR chemical shift of the iodine-substituted carbons.
5 mixture of the oxazinone 7 and the corresponding oxazolidinone (66% and 4% yield respectively).17 In each case, regioselectivity was determined using the diagnostic upfield 13C NMR chemical shift of the iodine-substituted carbons.
 | ||
| Scheme 2 Complementary cyclisations onto the central alkene of the hex-2-ene-1,6-diamine derivatives 1. The specific Ar groups used are shown in the products. | ||
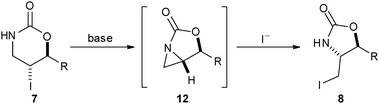
Treatment of the trichloroacetamide 4 with LiHMDS induced cyclisation to yield the oxazoline 5 which could be hydrolysed to give the differentially-protected diamine 6. In contrast, treatment of the oxazinone 7 with LiHMDS triggered an unexpected rearrangement to give the isomeric oxazolidinone 8. Presumably, deprotonation of 7 results in participation to yield the corresponding aziridine 12 which is then ring-opened by iodide (Scheme 3). The trans configuration of the oxazolidinone 8 was assigned by the characteristic18 coupling constant (4.5 Hz) between the ring protons. Although we are unaware of related rearrangements of oxazinones, aziridines related to the intermediate 12 have been prepared19 and ring-opened with nucleophiles.20
Palladium-catalysed aminoarylation also enabled cyclisation onto the central alkene of 1b and the differentially-protected 1c. With 5 mol% Pd(OAc)2, 10 mol% BINAP, Cs2CO3 in dioxane at 100 °C,21 cyclisation occurred as expected exclusively through the distal nitrogen to afford the corresponding pyrrolidines 10a–b and 11a–b with ∼85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 diastereoselectivity.
15 diastereoselectivity.
A range of bicyclic scaffolds was prepared from the cyclisation products 6 and 10a (Scheme 4). Thus, Boc-deprotection of 6, and reaction with CDI, yielded the bicyclic oxazolidinone 13. Similarly, 10a was converted into the related bicyclic scaffold 16. Alternatively, capping of the hydroxyl group of 6 by silylation, followed by Boc deprotection and LiHMDS-mediated cyclisation yielded the alternative bicyclic scaffold 14. Finally, tBuOK in THF triggered cyclisation of the hydroxy group of 6 (with displacement of trichloromethyl anion) to yield the oxazolidinone 15; similar cyclisations22 have been previously reported.
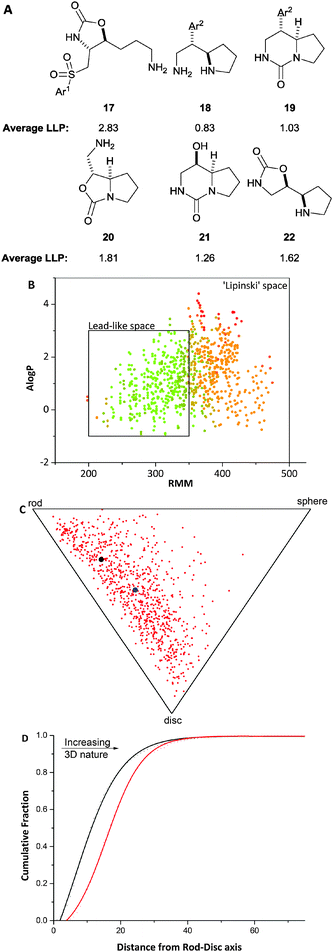
Overall, six diverse scaffolds were therefore prepared from the differentially-protected diamines 1. LLAMA was used to assess the value of the scaffolds 17–22 (Fig. 3). In each case, the enumerated library comprised compounds that had been decorated once or twice with exemplar medicinal chemistry capping groups.¶
Our approach yielded scaffolds that allow significant lead-like chemical space to be explored [average lead-likeness penalty: 1.57 (σ = 1.44) for our virtual library cf. 4.17 (σ = 3.17) for compounds from the ZINC database] (Panel B). In addition, the enumerated compounds are significantly more three-dimensional than a random 2% of the ZINC database (Panels C and D). Finally, the novelty assessment showed that the Murcko assembly (without alpha-atoms) of only one scaffolds was known (18 with Ar2 = 4-trifluoromethylphenyl) in the 2% selection of the ZINC database; however, the Murcko assembly with alpha atoms was not known for this scaffold, indicating that its substitution pattern is novel. We note that LLAMA may also be used prospectively to design scaffolds (e.g. specific Ar2 groups in 19) that are both novel and lead-like.
In conclusion, our synthetic approach exploited complementary cyclisations of hex-2-ene-1,6-diamine derivatives to yield a range of novel, lead-like small molecule scaffolds. The computational tool LLAMA can support the development of lead-oriented synthetic approaches by enabling assessment of the lead-likeness of alternative product scaffolds.
We acknowledge funding from EPSRC (EP/J00894X/1) and GSK for the development of LLAMA. We acknowledge support from the Innovative Medicines Initiative Joint Undertaking under grant number 115489, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (F97/2008–2013) and EFPIA companies' in kind contribution for scaffold synthesis. IC (Ignacio Colomer), SPM and AN contributed to the scaffold syntheses; CJE, PC, ZO, RD, IC (Ian Churcher), SPM and AN contributed to the development of LLAMA.
Notes and references
- (a) M. J. Waring, Bioorg. Med. Chem. Lett., 2009, 19, 2844 CrossRef CAS PubMed; (b) P. D. Leeson and B. Springthorpe, Nat. Rev. Drug Discovery, 2007, 6, 881 CrossRef CAS PubMed; (c) T. J. Ritchie and S. J. F. Macdonald, Drug Discovery Today, 2009, 14, 1011 CrossRef CAS PubMed; (d) M. C. Wenlock, R. P. Austin, P. Barton, A. M. Davis and P. D. Leeson, J. Med. Chem., 2003, 46, 1250 CrossRef CAS PubMed; (e) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed.
- (a) C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3 CrossRef CAS; (b) C. A. Lipinski, Drug Discovery Today, 2004, 1, 337 CrossRef CAS PubMed.
- A single metric has been developed to estimate drug-likeness by integrating a number of relevant parameters: G. R. Bickerton, G. V. Paolini, J. Besnard, S. Muresan and A. L. Hopkins, Nat. Chem., 2012, 4, 90 CrossRef CAS PubMed.
- (a) T. I. Oprea, A. M. Davis, S. J. Teague and P. D. Leeson, J. Chem. Inf. Comput. Sci., 2001, 41, 1308 CrossRef CAS PubMed; (b) E. Perola, J. Med. Chem., 2010, 53, 2986 CrossRef CAS PubMed; (c) M. M. Hann, Med. Chem. Commun., 2011, 2, 349 RSC; (d) G. M. Keserü and G. M. Makara, Nat. Rev. Drug Discovery, 2009, 8, 203 CrossRef PubMed.
- A. Nadin, C. Hattotuwagama and I. Churcher, Angew. Chem., Int. Ed., 2012, 51, 1114 CrossRef CAS PubMed.
- (a) A. Chuprina, O. Lukin, R. Demoiseaux, A. Buzko and A. Shivanyuk, J. Chem. Inf. Model., 2010, 50, 470 CrossRef CAS PubMed; (b) R. G. Doveston, P. Tosatti, M. Dow, D. J. Foley, H. Y. Li, A. J. Campbell, D. House, I. Churcher, S. P. Marsden and A. Nelson, Org. Biomol. Chem., 2015, 13, 859 RSC.
- A. H. Lipkus, Q. Yuan, K. A. Lucas, S. A. Funk, W. F. Bartelt III, R. J. Schenck and A. J. Trippe, J. Org. Chem., 2008, 73, 4443 CrossRef CAS PubMed.
- (a) P. MacLellan and A. Nelson, Chem. Commun., 2013, 49, 2383 RSC; (b) R. Doveston, S. P. Marsden and A. Nelson, Drug Discovery Today, 2014, 19, 813 CrossRef CAS PubMed.
- (a) D. J. Foley, R. G. Doveston, I. Churcher, A. Nelson and S. P. Marsden, Chem. Commun., 2015, 51, 11174 RSC; (b) T. James, I. Simpson, J. A. Grant, V. Sridharan and A. Nelson, Org. Lett., 2013, 15, 6094 CrossRef CAS PubMed; (c) T. James, P. MacLellan, G. M. Burslem, I. Simpson, J. A. Grant, S. Warriner, V. Sridharan and A. Nelson, Org. Biomol. Chem., 2014, 12, 2584 RSC.
- (a) M. Lüthy, M. C. Wheldon, C. Haji-Cheteh, M. Atobe, P. S. Bond, P. O'Brien, R. E. Hubbard and I. J. Fairlamb, Bioorg. Med. Chem., 2015, 23, 2680 CrossRef PubMed; (b) S. V. Ryabukhin, D. M. Panov, D. S. Granat, E. N. Ostapchuk, D. V. Kryvoruchko and O. O. Grygorenko, ACS Comb. Sci., 2014, 16, 146 CrossRef CAS PubMed; (c) A. Borisov, V. Voloshchuk, M. Nechayev and O. Grygorenko, Synthesis, 2013, 2413 CAS.
- The default set of capping groups is based on those that we have used in previous analyses (ref. 6b). See ESI†.
- G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887 CrossRef CAS PubMed.
- J. J. Irwin, T. Sterling, M. M. Mysinger, E. S. Bolstad and R. G. Coleman, J. Chem. Inf. Model., 2012, 52, 1757 CrossRef CAS PubMed.
- (a) W. H. B. Sauer and M. K. Schwarz, J. Chem. Inf. Comput. Sci., 2003, 43, 987 CrossRef CAS PubMed; (b) N. C. Firth, N. Brown and J. Blagg, J. Chem. Inf. Model., 2012, 52, 2516 CrossRef CAS PubMed.
- D. Robbins, A. F. Newton, C. Gignoux, J.-C. Legeay, A. Sinclair, M. Rejzek, C. A. Laxon, S. K. Yalamanchili, W. Lewis, M. A. O'Connell and R. A. Stockman, Chem. Sci., 2011, 2, 2232 RSC.
- For examples of iodocyclisations of N-Boc pentenamines, see: (a) K. Kamiyama, Y. Urano, S. Kobayashi and M. Ohno, Tetrahedron Lett., 1987, 28, 3123 CrossRef CAS; (b) S. G. Davies, G. Darren, M. Smyth and A. Chippindale, J. Chem. Soc., Perkin Trans. 1, 1999, 3089 RSC.
- J. M. Jordá-Gregori, M. E. González-Rosende, P. Cava-Montesinos, J. Sepúlveda-Arques, R. Galeazzi and M. Orena, Tetrahedron: Asymmetry, 2000, 11, 3769 CrossRef.
- A. G. Wee and D. D. McLeod, J. Org. Chem., 2003, 68, 6268 CrossRef CAS PubMed.
- For similar rearrangements of γ-lactams and cyclic ureas, see: (a) A. Sudau, W. Münch, J.-W. Bats and U. Nubbemeyer, Eur. J. Org. Chem., 2002, 3304 CrossRef CAS; (b) C. Agami, F. Amiot, F. Couty and L. Dechoux, Tetrahedron Lett., 1998, 39, 5373 CrossRef CAS.
- For examples for similar aziridines being ring-opening by a range of nucleophiles, see: (a) A. Padwa, A. C. Flick, C. A. Leverett and T. Stengel, J. Org. Chem., 2004, 69, 6377 CrossRef CAS PubMed; (b) S. Knapp and Y. Yu, Org. Lett., 2007, 9, 1359 CrossRef CAS PubMed.
- M. B. Bertrand, J. D. Neukom and J. P. Wolfe, J. Org. Chem., 2008, 73, 8851 CrossRef CAS PubMed.
- (a) R. Link, G. Subharekha, M. Gallant, S. J. Danishefsky, T. C. Chou and L. M. Ballas, J. Am. Chem. Soc., 1996, 118, 2825 CrossRef; (b) K. Stankov, M. Martinkov, J. Gonda, M. Bago, M. Piltov and G. Gönciov, Tetrahedron: Asymmetry, 2015, 26, 1394 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc03244c |
| ‡ The proportion has been estimated to 2.6% (ref. 5), 32% (ref. 6a) and 23% (ref. 6b) depending on the specific criteria and reference set used. |
| § LLAMA is available at: http://https://llama.leeds.ac.uk. |
| ¶ The scaffolds 17–19 already contain a variable group (Ar1 or Ar2) and were subjected to only one further decoration. |
| This journal is © The Royal Society of Chemistry 2016 |