 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly of a “double dynamic covalent” amphiphile featuring a glucose-responsive imine bond†
Xin
Wu
ab,
Xuan-Xuan
Chen
a,
Miao
Zhang
a,
Zhao
Li
a,
Philip A.
Gale
 b and
Yun-Bao
Jiang
*a
b and
Yun-Bao
Jiang
*a
aDepartment of Chemistry, College of Chemistry and Chemical Engineering, The MOE Key Laboratory of Spectrochemical Analysis and Instrumentation, and Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Xiamen University, Xiamen 361005, China. E-mail: ybjiang@xmu.edu.cn; Fax: +86 592 218 6405; Tel: +86 592 218 8372
bChemistry, University of Southampton, Southampton SO17 1BJ, UK
First published on 21st April 2016
Abstract
Glucose binding via boronate ester linkages selectively triggers imine bond formation between 4-formylphenylboronic acid and octylamine, leading to the formation of vesicular aggregates in aqueous solutions. This “double dynamic covalent assembly” allows the facile selective sensing of glucose against the otherwise serious interferant fructose, without the need to resort to synthetic effort.
The use of dynamic covalent bonds in the construction of complex molecular assemblies is a rapidly expanding area of research.1 Compared with noncovalent interactions that are weak and always exchanging, dynamic covalent bonds can function effectively in highly competitive media leading to significantly more stable assemblies that can be further stabilised “temporarily” (e.g. stabilising a hydrazone by increasing medium pH2) or “permanently” (e.g. reducing an imine to an amine3). Differing from the “permanent” covalent bonds used in organic synthesis, dynamic covalent bonds allow component exchange and can be highly responsive to environmental conditions such as temperature,4 pH,5 phase separation6 and molecular recognition events. Of particular interest is the responsiveness of dynamic covalent bonds to molecular recognition events. Many examples have been reported in which receptor structures were optimized through evolution of a library of assembling components in the presence of the substrate of interest as the template.7 While reported examples have focused on optimisation of the receptor structure from possible library members, little attention has been paid to the effect of substrate binding on the extent of dynamic covalent bond formation. In principle, substrate binding should be able to amplify the formation of originally weak dynamic covalent bonds that assemble the receptor. If molecular recognition between the receptor and the substrate occurs via another dynamic covalent bond (instead of commonly employed noncovalent interactions), a molecular assembly involving receptor assembly and receptor-substrate binding would form that results in simultaneous stabilisation of two or more dynamic covalent bonds. This could be an attractive step towards the creation of complex structures with potential applications such as sensing and drug delivery. Herein we report such a system, in which formation of an imine bond occurs to a small extent without a bound substrate, but is significantly and selectively amplified by glucose binding to an aldehyde moiety via boronate ester linkages to form a glucose bound supramolecular assembly (Fig. 1). The “dynamic covalent amphiphile” formed between 4-formylphenylboronic acid (4FBA), octylamine (C8AM) and glucose self-assemble into vesicular aggregates in aqueous solutions, allowing selective glucose sensing simply by mixing commercially available reagents.
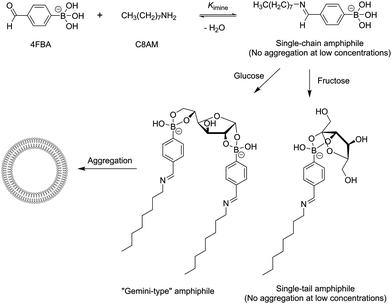 | ||
| Fig. 1 Proposed mechanism of glucose-induced aggregation of an in situ formed amphiphile that involves formation of two orthogonal dynamic covalent bonds. | ||
It has been well-established that monoboronic acids have an intrinsic preference for binding fructose selectively amongst the common monosaccharides, due to the abundance of its boronic acid-accessible β-furanose form.8 Selective sensing of glucose can be achieved by using diboronic acids that chelate glucose via binding two cis-diol moieties of its α-furanose forms.9 It has also been reported recently that glucose can induce aggregation of simple boronic acids due to its ability to crosslink two boronic acid molecules.10 We hypothesized that a glucose selective sensor can be as simple as an amphiphilic boronic acid, where the hydrophobic group can be attached to a hydrophilic boronic acid via a dynamic covalent linkage, preferably an imine bond11 due to its rapid kinetics. Glucose binding was expected to induce amphiphile aggregation, and as a result “indirectly” amplify the imine bond formation that is responsible for assembling the amphiphilic boronate ester. To test this idea, we chose to use simple components, 4-formylphenylboronic acid (4FBA) and octylamine (C8AM) (Fig. 1). The ability of 4FBA to form an imine bond with C8AM and a boronate ester linkage with saccharides in aqueous solutions has been confirmed by 1H NMR studies (Fig. S4, ESI†).
To allow imine bond formation while ensuring water-solubility of all components, we carried out the self-assembly studies at pH 10.5 (with 100 mM sodium carbonate buffer). Under these conditions, C8AM (pKa 10.6512) is partially protonated and maintains water solubility at 3 mM. 4FBA (pKa 7.413) exists completely in its anionic form which maximizes its saccharide binding affinity. When 4FBA (3 mM) and C8AM (3 mM) were mixed at pH 10.5, the solution remained clear and transparent (Fig. 2a). In the presence of glucose (5 mM), however, the solution becomes increasingly turbid over the course of 30 min, indicating that amphiphile aggregation took place (Fig. 2a). With galactose (5 mM) used as the saccharide component, a lower degree of turbidity was observed, whereas with fructose (5 mM) the solution remained transparent (Fig. 2a).
To further investigate amphiphilic aggregation, we employed Nile red, a hydrophobic environment-sensitive fluorescent dye. In aqueous solutions Nile red is non-fluorescent, but in the presence of amphiphile aggregates (e.g. micelles and vesicles), Nile red can partition into the hydrophobic region of the aggregates so becoming strongly fluorescent. Mixtures of 4FBA (3 mM) and C8AM (3 mM) in the absence and presence of varying concentrations of saccharides were incubated for 30 min, treated with a methanol solution of Nile red, and subject to fluorescence measurements. The results are shown in Fig. 2b. Very weak fluorescence from Nile red was observed without saccharides or with fructose (0.1–10 mM), confirming that little or no amphiphile aggregation occurred. In contrast, the presence of glucose and galactose (to a lesser extent) led to dramatic enhancement of Nile red fluorescence. These results agree well with those of the turbidity assay, confirming that under the described conditions little or no aggregation occurred without saccharides or with fructose, but glucose and galactose induced self-assembly of the amphiphile formed between C8AM and 4FBA. Assembly of a control compound 4-formylbenzoic acid with C8AM was also examined using the Nile red assay, which showed no saccharide-dependence in the amphiphile aggregation (Fig. S3, ESI†). This confirmed the role of saccharide binding to the boronic acid group in the 4FBA/C8AM system. It is well known that the α-furanose forms of glucose and galactose can simultaneously bind two boronic acid moieties whereas the β-fructofuranose can only bind a single boronic acid moiety. Therefore binding of glucose and galactose can lead to formation of “Gemini-type” amphiphiles, which have a higher ability of aggregation compared to “single-tail” amphiphiles formed with 4FBA and C8AM, or additionally fructose (Fig. 1). This explains why induction of aggregation was observed only with glucose and galactose. The weaker ability of galactose to induce aggregation is probably due to the unfavorable orientation of two cis-diol moieties in the α-galactosefuranose as compared with those in α-glucofuranose.14 It should be noted that although the fluorescence intensity (which depends on the amount of Nile red) leveled off at 5 mM of glucose and galactose, the formation of amphiphile aggregates is still far from saturation, as will be demonstrated in the imine formation study below.
Since glucose and galactose (to a lesser extent) induce aggregate formation, it is expected that they also influence the equilibrium of imine bond formation which should depend on the aggregation process. It has been reported by van Esch and coworkers that amphiphile aggregation can drive imine bond formation,15 and this is likely to be true for this system as well. We employed 1H NMR spectroscopy to measure imine bond formation. Characteristic imine proton NMR resonances at 8.3 ppm were observed in the absence and presence of saccharides, providing direct evidence of the imine bond formation (Fig. S6–S9, ESI†). Although using conventional liquid-state NMR techniques, the NMR signals from the aggregates cannot be quantified due to broadening (Fig. S7 and S9, ESI†), indirect measurement of the percentage of imine formation is possible by calculation of 4FBA consumption by integration of its 1H NMR resonances. To enable this calculation, we added N,N-dimethylformamide (DMF, equal amount to 4FBA and C8AM) as an internal reference. By comparing integrations of the 1H NMR signals of aldehyde (CHO) and DMF, the percentage of imine formation was calculated and is summarized in Table 1.
| Condition | Blank | Glucose (5 mM) | Fructose (5 mM) | Galactose (5 mM) |
|---|---|---|---|---|
| Imine formation | 7% | 38% | 12% | 26% |
Interestingly, imine bond formation was indeed found to be enhanced dramatically by glucose binding and to a lesser extent by galactose binding, whereas not as significantly with fructose that cannot promote amphiphile aggregation and its effect is likely due to a minor influence on the intrinsic reactivity of the aldehyde group. These results can be explained by (i) amphiphile aggregation shifts the equilibrium of the imine bond formation in the forward direction, and (ii) binding of glucose and galactose promotes amphiphile aggregation due to the formation of “Gemini-type” amphiphiles. Notably, glucose binding via the boronate ester linkage exerted an influence on the imine bond despite the spatial separation between the two dynamic covalent bonds. This is possibly because of the supramolecular aggregation that requires and stabilises both the imine bond and the boronate ester linkages (with “divalent” binder glucose). This “indirect” interplay is conceptually distinct from the known synergistic binding of 2-formylphenylboronic acid (2FBA) to an amine and a cis-diol component,16 which is due to the cis-diol binding making the boron center more acidic17 thus enhancing the boron–nitrogen interaction.
The aggregates formed with glucose were further characterised by transmission electron microscopy (TEM) and dynamic light scattering (DLS) techniques (Fig. 2c and d). The spherical morphology and dark exterior shown by the TEM image revealed that the aggregates formed are vesicles. DLS measurements revealed an average hydrodynamic diameter (Dh) of 678 nm. The anionic nature of the aggregates resulting from the anionic boronate head groups (Fig. 1) was supported by the negative value of the measured zeta potential of −33.3 mV.
This system may be used for glucose sensing via the appearance of the solution turbidity which can be detected by the naked eye or quantified by measuring light scattering using an absorption or fluorescence spectrometer. Alternatively, the incorporation of Nile red allows sensitive fluorescence sensing of glucose at sub-mM concentrations. We were interested in testing the ability of this glucose sensing ensemble to tolerate the presence of saccharide interferents. Promisingly, the presence of 0.2 mM of fructose or galactose resulted in little interference with sensing of 1 mM glucose (Fig. S14, ESI†), a significant improvement compared with other reported systems based on self-assembly,10b,c although fructose and galactose at higher concentrations did lead to significant interference. Note that the boronic acid component 4FBA has a 24-fold fructose/glucose binding selectivity.13 The improvement of glucose selectivity demonstrated in the ensemble highlights the role of two synergistically acting dynamic covalent bonds coupled to supramolecular polymerization.
In summary, we have demonstrated in a simple system that the equilibrium of a dynamic covalent bond that assembles a receptor can respond to a molecular recognition event via a different dynamic covalent bond, and such an assembly has been used for sensing applications. A mixture of 4FBA, C8AM and glucose formed a dynamic “Gemini-type” amphiphile that self-assembled to form vesicular aggregates which features simultaneous formation of an imine bond and boronate ester linkages with glucose. Interestingly, there is a large spatial separation between the two dynamic covalent bonds, and their mutual influence is made possible because of the amphiphile aggregation and multivalent binding with glucose. Our study also relates to the interesting question of integrating dynamic covalent chemistry to supramolecular polymerization.18 The reported system allows glucose sensing simply by mixing commercially available reagents, representing the first example that the intrinsic fructose over glucose selectivity of boronic acids can be overcome without resorting to synthesis. This suggests that the structural complexity required for creating selective synthetic receptors or other functional materials can be achieved by in situ dynamic covalent assembly of simple components.
This work was supported by the NSF of China (grants 21275121, 21435003, 91427304, 21521004), and the Program for Changjiang Scholars and Innovative Research Team in University, administrated by the MOE of China (grant IRT13036). XW thanks the China Scholarship Council and the University of Southampton for a PhD studentship. PAG thanks the Royal Society and the Wolfson Foundation for a Research Merit Award. We thank Wenqiang Zhang and Hualu Zhou (Xiamen University) for their help with TEM, and Dr Neil J. Wells (University of Southampton) for performing 11B NMR.
Notes and references
- (a) A. Wilson, G. Gasparini and S. Matile, Chem. Soc. Rev., 2014, 43, 1948–1962 RSC; (b) J. Li, P. Nowak and S. Otto, J. Am. Chem. Soc., 2013, 135, 9222–9239 CrossRef CAS PubMed; (c) S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef.
- L. A. Tatum, X. Su and I. Aprahamian, Acc. Chem. Res., 2014, 47, 2141–2149 CrossRef CAS PubMed.
- J. Mosquera, S. Zarra and J. R. Nitschke, Angew. Chem., Int. Ed., 2013, 53, 1556–1559 CrossRef PubMed.
- K. Imato, T. Ohishi, M. Nishihara, A. Takahara and H. Otsuka, J. Am. Chem. Soc., 2014, 136, 11839–11845 CrossRef CAS PubMed.
- C. Wang, G. Wang, Z. Wang and X. Zhang, Chem. – Eur. J., 2011, 17, 3322–3325 CrossRef CAS PubMed.
- N. Hafezi and J.-M. Lehn, J. Am. Chem. Soc., 2012, 134, 12861–12868 CrossRef CAS PubMed.
- (a) L. I. James, J. E. Beaver, N. W. Rice and M. L. Waters, J. Am. Chem. Soc., 2013, 135, 6450–6455 CrossRef CAS PubMed; (b) R. T. S. Lam, A. Belenguer, S. L. Roberts, C. Naumann, T. Jarrosson, S. Otto and J. K. M. Sanders, Science, 2005, 308, 667–669 CrossRef CAS PubMed.
- (a) X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS PubMed; (b) X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 8032–8048 RSC; (c) T. D. James, M. D. Phillips and S. Shinkai, Boronic Acids in Saccharide Recognition, The Royal Society of Chemistry, Cambridge, UK, 2006 Search PubMed.
- (a) M. Bielecki, H. Eggert and J. C. Norrild, J. Chem. Soc., Perkin Trans. 2, 1999, 449–456 RSC; (b) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed. Engl., 1994, 33, 2207–2209 CrossRef.
- (a) Y. Tsuchido, Y. Sakai, K. Aimu, T. Hashimoto, K. Akiyoshi and T. Hayashita, New J. Chem., 2015, 39, 2620–2626 RSC; (b) Y.-J. Huang, W.-J. Ouyang, X. Wu, Z. Li, J. S. Fossey, T. D. James and Y.-B. Jiang, J. Am. Chem. Soc., 2013, 135, 1700–1703 CrossRef CAS PubMed; (c) Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660–663 CrossRef CAS PubMed.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- C. W. Hoerr, M. R. McCorkle and A. W. Ralston, J. Am. Chem. Soc., 1943, 65, 328–329 CrossRef CAS.
- N. J. Gutierrez-Moreno, F. Medrano and A. K. Yatsimirsky, Org. Biomol. Chem., 2012, 10, 6960–6972 CAS.
- H. Eggert, J. Frederiksen, C. Morin and J. C. Norrild, J. Org. Chem., 1999, 64, 3846–3852 CrossRef CAS.
- (a) D. Janeliunas, P. van Rijn, J. Boekhoven, C. B. Minkenberg, J. H. van Esch and R. Eelkema, Angew. Chem., Int. Ed., 2013, 52, 1998–2001 CrossRef CAS PubMed; (b) C. B. Minkenberg, F. Li, P. van Rijn, L. Florusse, J. Boekhoven, M. C. A. Stuart, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 3421–3424 CrossRef CAS PubMed; (c) C. B. Minkenberg, L. Florusse, R. Eelkema, G. J. M. Koper and J. H. van Esch, J. Am. Chem. Soc., 2009, 131, 11274–11275 CrossRef CAS PubMed.
- (a) M. Hutin, G. Bernardinelli and J. R. Nitschke, Chem. – Eur. J., 2008, 14, 4585–4593 CrossRef CAS PubMed; (b) Y. Pérez-Fuertes, A. M. Kelly, A. L. Johnson, S. Arimori, S. D. Bull and T. D. James, Org. Lett., 2006, 8, 609–612 CrossRef PubMed.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- Z. Yu, F. Tantakitti, T. Yu, L. C. Palmer, G. C. Schatz and S. I. Stupp, Science, 2016, 351, 497–502 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, fluorescence spectra, NMR spectra and competition studies. See DOI: 10.1039/c6cc03167f |
| This journal is © The Royal Society of Chemistry 2016 |

