 Open Access Article
Open Access ArticleMicroporous polyurethane material for size selective heterogeneous catalysis of the Knoevenagel reaction†
Sandeep Kumar
Dey‡
,
Nader
de Sousa Amadeu
and
Christoph
Janiak
*
Institut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de; Fax: +49-211-81-12287; Tel: +49-211-81-12286
First published on 20th May 2016
Abstract
The first polyurethane material which is microporous (BET surface area of 312 m2 g−1) is prepared by solvothermal synthesis and acts as highly efficient and recyclable heterogeneous catalyst in the Knoevenagel condensation showing size selectivity, and in the Henry reaction showing substrate selectivity under mild reaction conditions.
The development of functional porous organic frameworks (POFs) assembled from simple molecular building blocks has gained considerable attention in recent times,1 mainly because of their potential applications in gas storage and separation,2 catalysis,3 optoelectronics4 and nanotechnology.5 Considering a number of covalent linkages that can be derived by applying different synthetic reactions of a wide variety of molecular building blocks, it has become increasingly important to develop new functional POFs having high specific surface area, catalytically active pore surface, and remarkable chemical and thermal stability. Although impressive progress has been achieved in the field of heterogeneous catalysis using modified zeolites and metal–organic frameworks (MOFs),6 improving the functions of the pores in POFs for better catalytic performance and selectivity still remains a major challenge.
There has been, so far, no report on porous polyurethanes (Scheme 1) as catalysts, to the best of our knowledge.
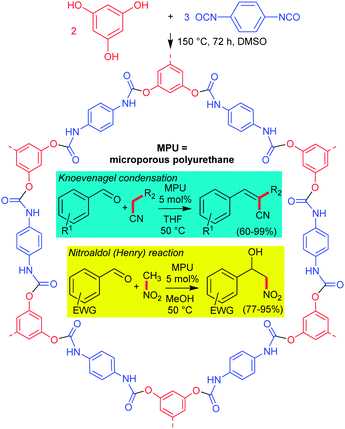 | ||
| Scheme 1 Solvothermal synthesis of the microporous polyurethane (MPU) material, with the MPU catalysed Knoevenagel condensation reaction (R1 = various electron donating and withdrawing substituents, see Table 1 and R2 = –CN/–COOMe) and the nitroaldol (Henry) reaction (EWG = electron withdrawing group, see Table 2) (product yields in parentheses). | ||
The Knoevenagel condensation (Scheme 1) is one of the most useful and widely employed carbon–carbon bond formation reactions enjoying widespread application in synthetic organic chemistry for obtaining fine chemicals and heterocyclic compounds of biological importance.7 Several amine and amide functionalized MOFs have been employed as heterogeneous solid catalysts to promote both the Knoevenagel and Henry reactions and thus, addressing the problems of recyclability and high loading common with homogeneous base catalysts such as alkali metal hydroxides and organic amines.8 Further, a wide range of other porous solid catalysts have also been investigated for Knoevenagel reaction.9 Yet, only a couple of examples of POF-catalysed Knoevenagel reactions are known in literature.10 Its variant the nitroaldol (Henry) reaction (Scheme 1) has not been reported with POF catalysts, to the best of our knowledge.
Porous polyurethanes have received considerable attention in recent years, because of their high elastance, biocompatibility and biodegradability essential for soft tissue engineering applications.11 They have also been used in enzyme immobilization and in biosensor applications.12 In our attempt to assess the, so far unknown catalytic activities of porous polyurethane, herein we report a new microporous polyurethane (MPU), synthesized by an unprecedented solvothermal route for MPUs, based on the condensation of phloroglucinol and 1,4-phenylenediisocyanate and its catalytic efficiency in the Knoevenagel and Henry reactions under mild conditions (Scheme 1). Due to its mild basic nature and ability to form hydrogen bonds, the urethane (carbamate) group can act as a catalytic driving force to prompt several important base-catalysed reactions.
The microporous polyurethane (MPU) material was synthesized solvothermally by the condensation of phloroglucinol and 1,4-phenylenediisocyanate in dimethyl sulfoxide at 150 °C (Scheme 1, see ESI† for details). The successful formation of the MPU was verified by Fourier transform infrared (FT-IR) and solid-state 13C CP/MAS NMR spectroscopy. The FT-IR spectrum of the MPU showed disappearance of the stretching band of isocyanate (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 2274 cm−1 and concomitant emergence of the carbonyl (C
O) at 2274 cm−1 and concomitant emergence of the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching band at 1633 cm−1 (Fig. S2, ESI†) indicating complete condensation between 1,4-phenylenediisocyanate and phloroglucinol. Solid-state 13C NMR spectrum further confirmed the presence of carbonyl carbon of the urethane moiety at δ = 156 ppm, whereas other peaks at 150, 134, 125, and 109 ppm were assigned to carbon atoms of the aryl rings (Fig. S3, ESI†).
O) stretching band at 1633 cm−1 (Fig. S2, ESI†) indicating complete condensation between 1,4-phenylenediisocyanate and phloroglucinol. Solid-state 13C NMR spectrum further confirmed the presence of carbonyl carbon of the urethane moiety at δ = 156 ppm, whereas other peaks at 150, 134, 125, and 109 ppm were assigned to carbon atoms of the aryl rings (Fig. S3, ESI†).
The PXRD pattern of the activated MPU (Fig. S4, ESI†) indicated the partial ordered nature of the framework as reflected from the presence of several peak maxima in the diffractogram, notably in the 2θ range of 20° to 30° at 20.3° (d = 4.37 Å), 21.4° (d = 4.14 Å), 26.2° (d = 3.39 Å), 27.8° (d = 3.20 Å) and 29.9° (d = 2.99 Å). SEM images revealed that the MPU crystallizes as small proliferated flakes, which are aggregated into an overall sponge like morphology (Fig. S5, ESI†). The SEM images also showed the presence of scarcely distributed spherical particles, a likely indication towards the initial formation of the MPU spheres which eventually burst out forming the proliferated flakes during the solvothermal synthesis. Energy dispersive X-ray spectrometric (EDX) measurement showed the presence of sulfur which strongly suggests the inclusion of DMSO molecules within the pores of the framework (Fig. S6, ESI†). CHNS analysis revealed the presence of ∼2.0 wt% of sulfur in the activated MPU, which accounts for ∼6.6 wt% of DMSO of the total weight of the polymer (Table S2, ESI†). This result was supported by the thermogravimetric analysis (TGA) of the activated MPU, which showed 6.2% mass loss beginning at 180 °C till 280 °C, beyond which the polymer starts to decompose (Fig. S7, ESI†).
A nitrogen sorption measurement at 77 K showed permanent microporosity of the MPU with a BET surface area of 312 m2 g−1. The nitrogen sorption isotherm is a composite of Types I and II with an H4 hysteresis loop (Fig. 1a). The pronounced gas uptake at P/P0 < 0.10 is associated with the filling of micropores. H4 loops are often found with aggregated crystals of zeolites, some mesoporous zeolites, and micro-mesoporous carbons.13 Non-local density functional theory (NLDFT)14 pore size distribution using a slit pore model indicates that a significant fraction of the pores of the MPU originates from pores with a diameter less than 20 Å (micropores by definition).13 Accordingly, the pore size distribution curve showed micropores centred at a diameter of 12 Å and 16 Å, and a small fraction of mesopores between 20–40 Å (Fig. 1b). Larger micropores of 16 Å give a possible indication towards the formation of hexagonal pore structure in the MPU material, while smaller micropores of 12 Å could be the result of hexagonal pore shrinking due to the inclusion of DMSO within the porous framework. The total pore volume was calculated to be 0.30 cm3 g−1.
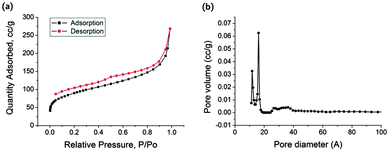 | ||
| Fig. 1 (a) Nitrogen sorption isotherm at 77 K, and (d) NLDFT pore size distribution curve of the activated MPU using a slit pore model. | ||
Over the past few years, the use of POFs as heterogeneous catalyst for various organic reactions has been of continuous growing interest.3 For the Knoevenagel reaction, two 3D microporous imine-COFs (BF-COFs) have recently been shown to catalyse the condensation reaction in benzene with selectivity for benzaldehyde only.10a Further, an amide functionalized microporous organic polymer (Am-MOP) has also been shown to catalyse the Knoevenagel reaction in THF, albeit giving only three examples of benzaldehyde derivatives.10b A POF-catalysed Henry reaction has not been reported till date, to the best of our knowledge.
Encouraged by these results, the new MPU was assessed for its catalytic activity in the Knoevenagel reaction using a model reaction, wherein benzaldehyde reacts with malononitrile in the presence of 5 mol% of activated MPU in tetrahydrofuran at 50 °C to give 98% yield of the desired α,β-unsaturated product within 14 h. In the absence of the MPU catalyst, only 5% desired product was formed under the same optimal reaction-condition. The reaction when carried out at room temperature (25 °C) did not proceed to completion, and yielded 90% of desired product even after 24 h. The quantitative analysis of the Knoevenagel product formation was monitored by 1H NMR spectroscopy at regular intervals of time. The detailed kinetic study showed that the MPU catalysed Knoevenagel reaction is completed within 14 h at 50 °C in THF (Fig. S15, ESI†).
In general, excellent conversions above 95% to the desired products were observed for most of the aromatic aldehyde substrates except for 9-anthracenecarboxaldehyde and biphenyl-4-carboxaldehyde (Table 1). This suggests that the reaction occurs within the pores of the MPU which cannot possibly accommodate substrates of larger dimensions. Nevertheless, if the reaction has occurred, the products might have been trapped within the pores due to their larger size as compared to the reactants and thus, no more reaction could occur. It should be noted that, a comparatively decreased yield of 80% has been observed with 2-naphthaldehyde given its larger molecular size (5.6 Å × 7.5 Å) as compared to benzaldehyde (5.0 Å × 5.6 Å) and its derivatives. While the molecular sizes of biphenyl-4-carboxaldehyde (4.3 Å × 10.4 Å) and 9-anthracenecarboxaldehyde (6.4 Å × 9.6 Å) are comparatively smaller than the larger micropores (16 Å) of the MPU, but their Knoevenagel products having larger molecular dimensions may not get out of the micropores if the reaction has occurred.
| Aromatic aldehydes | Yield (%) [TON]c of α,β-unsaturated product | |||
|---|---|---|---|---|
| R2 = –CNa | R2 = –COOMeb | |||
| a General conditions: aldehyde (1.0 mmol), malononitrile (72 mg, 1.1 mmol) in the presence of 5 mol% (18 mg) of MPU in tetrahydrofuran at 50 °C. b General conditions: aldehyde (1.0 mmol), methylcyanoacetate (110 mg, 1.1 mmol) in the presence of 5 mol% (18 mg) of MPU in tetrahydrofuran at 50 °C. c Yields corresponding also to aldehyde conversion are based on 1H NMR analysis of the isolated product obtained after 14 h of reaction time (Fig. S21–S49, ESI). TON (turnover number) = moles of desired product formed/moles of catalyst. | ||||
| Benzaldehyde | 98 | [19.6] | 60 | [12.0] |
| 4-Nitrobenzaldehyde | 99 | [19.8] | 95 | [19.0] |
| 3-Nitrobenzaldehyde | 99 | [19.8] | 95 | [19.0] |
| 2-Nitrobenzaldehyde | 99 | [19.8] | 90 | [18.0] |
| 4-Carboxybenzaldehyde | 99 | [19.8] | 86 | [17.2] |
| 4-Chlorobenzaldehyde | 97 | [19.4] | 89 | [17.8] |
| 4-Fluorobenzaldehyde | 98 | [19.6] | 77 | [15.4] |
| 4-Tolualdehyde | 95 | [19.0] | 63 | [12.6] |
| 4-Anisaldehyde | 99 | [19.8] | 45 | [9.0] |
| 2-Naphthalenealdehyde | 80 | [16.0] | 75 | [15.0] |
| 9-Anthracenealdehyde | 0 | [0] | 0 | [0] |
| Biphenyl-4-carboxaldehyde | 0 | [0] | 0 | [0] |
It is important to mention here that the reaction between benzaldehyde derivatives and malononitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio) can proceed without any catalyst in methanol/ethanol at 50 °C within 10 h (yield >90%). However, the background reaction is comparatively much slower in the case of the reaction between benzaldehyde derivatives and methylcyanoacetate (1
1.1 molar ratio) can proceed without any catalyst in methanol/ethanol at 50 °C within 10 h (yield >90%). However, the background reaction is comparatively much slower in the case of the reaction between benzaldehyde derivatives and methylcyanoacetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio) in methanol, and the yields are significantly improved using 5 mol% the MPU catalyst (Table S4, ESI†). High background reaction rate has also been observed in acetonitrile (MeCN) for the reaction between benzaldehyde and malononitrile giving a yield of 55% within 10 h at 50 °C. These observations suggested that protic solvents and nitrogen containing aprotic solvents (such as MeCN and DMF) are not generally suitable for catalyst evaluation in the Knoevenagel reaction. Recent studies have shown that DMF as well as water can significantly promote the Knoevenagel condensation with quantitative yield.15
1.1 molar ratio) in methanol, and the yields are significantly improved using 5 mol% the MPU catalyst (Table S4, ESI†). High background reaction rate has also been observed in acetonitrile (MeCN) for the reaction between benzaldehyde and malononitrile giving a yield of 55% within 10 h at 50 °C. These observations suggested that protic solvents and nitrogen containing aprotic solvents (such as MeCN and DMF) are not generally suitable for catalyst evaluation in the Knoevenagel reaction. Recent studies have shown that DMF as well as water can significantly promote the Knoevenagel condensation with quantitative yield.15
Further, the model Knoevenagel reaction performed in the presence of 5 mol% of homogeneous base catalysts such as, pyrrole and piperidine/pyridine, in THF at 50 °C yielded 67% and 98% respectively (Fig. S56 and S57, ESI†), of the desired product within 10 h. Also, imidazole/2-methylimidazole acts as a highly efficient homogeneous catalyst in the Knoevenagel reaction.16 However, unlike heterogeneous solid catalysts, these homogeneous catalysts could not be recycled and reused, and therefore they are practically undesirable.
The catalytic activity of the MPU has also been assessed in the Knoevenagel condensation of methylcyanoacetate with aromatic aldehydes under the same optimal reaction conditions in THF. It has been observed that the yields are significantly reduced for benzaldehyde (60%) and its derivatives with electron donating methyl and methoxy groups (Table 1). Whereas, excellent conversions above 80% have been observed for most of the benzaldehyde derivatives with electron withdrawing groups. As expected, reaction of methylcyanoacetate with 9-anthracenecarboxaldehyde and biphenyl-4-carboxaldehyde yielded no desired product due to their larger molecular size. These results strongly suggest that the MPU catalysed Knoevenagel reactions are closely dependent on pore size.
Increasing the molar reagent ratio of benzaldehyde and malononitrile to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 5 mol% of MPU at 50 °C in THF yielded 99% of the desired product after 8 h (Fig. S16, ESI†). Thus, the reagent ratio is also an important factor that should also be taken into consideration in the MPU catalysed Knoevenagel condensation.
2 with 5 mol% of MPU at 50 °C in THF yielded 99% of the desired product after 8 h (Fig. S16, ESI†). Thus, the reagent ratio is also an important factor that should also be taken into consideration in the MPU catalysed Knoevenagel condensation.
To further explore the catalytic efficiency of the MPU towards other C–C bond formation reactions, we have studied the Henry (nitroaldol) reaction of several aromatic aldehydes with nitromethane in the presence of 5 mol% of activated MPU in methanol at 50 °C (Table 2). It was observed that, excellent conversions above 90% to the desired β-nitroalkanol products were obtained with nitrobenzaldehyde(s), 4-cyanobenyaldehyde and 4-carboxybenzaldehyde within 10 h (Fig. S17, ESI†). However, no reaction has occurred with benzaldehyde, 2-naphthaldehyde, 4-anisaldehyde and biphenyl-4-carboxaldehyde under the same optimal reaction conditions. In general, the substrate scope of the MPU catalysed Henry reaction is limited to only benzaldehyde derivatives with electron withdrawing groups, however with significantly high yields (Table 2). Unlike the Knoevenagel reaction, the nitroaldol reaction between 4-nitrobenzaldehyde and nitromethane did not proceed in THF/MeCN signifying the importance of protic solvent in the MPU catalysed Henry reaction.
| Aromatic aldehydes | Yield (%) [TON] of β-nitroalkanola,b | |
|---|---|---|
| a General conditions: aldehyde (1.0 mmol), nitromethane (610 mg, 10 mmol) in the presence of 5 mol% (18 mg) of activated MPU in methanol at 50 °C. b Yields, corresponding also to aldehyde conversion are based on 1H NMR analysis of the isolated product obtained after 10 h of reaction time (Fig. S50–S55, ESI). TON (turnover number) = moles of desired product formed/moles of catalyst. | ||
| Benzaldehyde | 0 | [0] |
| 4-Nitrobenzaldehyde | 94 | [18.8] |
| 3-Nitrobenzaldehyde | 95 | [19.0] |
| 2-Nitrobenzaldehyde | 94 | [18.8] |
| 4-Cyanobenzaldehyde | 97 | [19.4] |
| 4-Carboxybenzaldehyde | 90 | [18.0] |
| 4-Chlorobenzaldehyde | 77 | [15.2] |
| 4-Tolualdehyde | 20 | [4.0] |
Hot filtration tests have been performed for the Knoevenagel and Henry reactions and the possibility of leaching of some of the active sites from the solid catalyst to the liquid phase has been ruled out (see ESI† for details).
The catalyst was recycled and used for two consecutive runs with no significant loss in activity for the Knoevenagel reaction between 4-nitrobenzaldehyde with malononitrile and for the Henry reaction between 4-nitrobenzaldehyde with nitromethane (Fig. S18, see ESI† for details). The recovered catalyst has been characterized by FT-IR, PXRD, SEM, CHNS analysis and nitrogen sorption measurements (Fig. S11–S14 and Tables S1, S2, ESI†). No significant changes have been observed in the FT-IR, PXRD, SEM and CHNS analysis data of the recycled MPU, only a slight decrease in BET specific surface area has been observed after the first and second catalytic run.
In conclusion, we have reported a catalytically active new microporous polyurethane material with sponge like morphology obtained by solvothermal synthesis in DMSO. Due to its confined microporosity and the presence of mildly basic urethane (carbamate) functional groups along the pore walls, the MPU showed catalytic efficiency with pronounced size selectivity in the Knoevenagel condensation of aromatic aldehydes with malononitrile or methylcyanoacetate. In the related nitroaldol (Henry) reaction involving nitromethane as the nucleophile, the catalyst showed high substrate selectivity as significant yields of β-nitroalkanol product could only be obtained with benzaldehyde derivatives having electron withdrawing groups. Overall, this work represents the first example of heterogeneous catalysis by a porous polyurethane material which will encourage the development of new functional porous polyurethanes for the catalysis of several other important reactions.
Notes and references
- S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC; X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010 RSC; Z. Xiang, D. Cao and L. Dai, Polym. Chem., 2015, 6, 1896 RSC.
- B. G. Hauser, O. K. Farha, J. Exley and J. T. Hupp, Chem. Mater., 2013, 25, 12 CrossRef CAS; W. Lu, D. Yuan, D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Braese, J. Guenther, J. Bluemel, R. Krishna, Z. Li and H.-C. Zhou, Chem. Mater., 2010, 22, 5964 CrossRef; A. Bhunia, I. Boldog, A. Möller and C. Janiak, J. Mater. Chem. A, 2013, 1, 14990 Search PubMed; A. Bhunia, V. Vasylyeva and C. Janiak, Chem. Commun., 2013, 49, 3961 RSC.
- P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819 CrossRef CAS; Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083 RSC.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2008, 120, 8958 CrossRef CAS; S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2009, 48, 5439 CrossRef PubMed; E. L. Spitler and W. R. Dichtel, Nat. Chem., 2010, 2, 672 CrossRef PubMed; X. Ding, L. Chen, Y. Honsho, X. Feng, O. Saengsawang, J. Guo, A. Saeki, S. Seki, S. Irle, S. Nagase, V. Parasuk and D. Jiang, J. Am. Chem. Soc., 2011, 133, 14510 CrossRef PubMed.
- D. F. Perepichka and F. Rosei, Science, 2009, 323, 216 CrossRef CAS PubMed; J. F. Dienstmaier, D. D. Medina, M. Dogru, P. Knochel and T. Bein, ACS Nano, 2012, 6, 7234 CrossRef PubMed; A. Lyapin, S. Reichlmaier, W. M. Heckl and M. Lackinger, ACS Nano, 2011, 5, 9737 CrossRef PubMed.
- J. Shi, Y. Wang, W. Yang, Y. Tang and Z. Xie, Chem. Soc. Rev., 2015, 44, 8877 RSC; A. Dhakshinamoorthy, M. Opanasenko, J. Cejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509 Search PubMed; A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804 RSC; J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC.
- F. Freeman, Chem. Rev., 1980, 80, 329 CrossRef CAS; L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef PubMed.
- S. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 2607 CrossRef CAS PubMed; Q. R. Fang, S. Gu, J. Zheng, Z. B. Zhuang, S. L. Qiu and Y. S. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878 CrossRef PubMed; J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef PubMed; U. P. N. Tran, K. K. A. Le and N. T. S. Phan, ACS Catal., 2011, 1, 120 CrossRef; A. Karmakar, C. L. Oliver, S. Roy and L. Öhrströma, Dalton Trans., 2015, 44, 10156 RSC; A. Karmakar, S. Hazra, M. Fátima, C. G. da Silva, A. Paul and A. J. L. Pombeiro, CrystEngComm, 2016, 18, 1337 RSC.
- R. Xing, H. Wu, X. Li, Z. Zhao, Y. Liu, L. Chen and P. Wu, J. Mater. Chem., 2009, 19, 4004 RSC; Z. Gao, J. Zhou, F. Cui, Z. H. Yan Zhu and J. Shi, Dalton Trans., 2010, 39, 11132 RSC; F. Shang, J. Sun, S. Wu, Y. Yang, Q. Kan and J. Guan, Microporous Mesoporous Mater., 2010, 134, 44 CrossRef CAS; S. H. Zhao, X. J. Wang and L. W. Zhang, RSC Adv., 2013, 3, 11691 RSC; A. Villa, J. P. Tessonnier, O. Majoulet, D. S. Su and R. Schlogt, ChemSusChem, 2010, 3, 241 CrossRef PubMed; J. Xu, Y. Wang, J.-K. Shang, Q. Jiang and Y.-X. Li, Catal. Sci. Technol. 10.1039/c5cy01747e; J. Xu, T. Chen, J.-K. Shang, K.-Z. Long and Y.-X. Li, Microporous Mesoporous Mater., 2015, 211, 105 CrossRef; N. Kan-nari, S. Okamura, S.-I. Fujita, J.-I. Ozaki and M. Arai, Adv. Synth. Catal., 2010, 352, 1476 CrossRef.
- (a) Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878 CrossRef CAS PubMed; (b) V. M. Suresh, S. Bonakala, H. S. Atreya, S. Balasubramanian and T. K. Maji, ACS Appl. Mater. Interfaces, 2014, 6, 4630 CrossRef CAS PubMed.
- J. Guan, K. L. Fujimoto, M. S. Sacks and W. R. Wagner, Biomaterials, 2005, 26, 3961 CrossRef CAS PubMed; Y.-C. Wang, F. Fang, Y.-K. Wu, X.-L. Ai, T. Lan, R.-C. Liang, Y. Zhang, N. M. Trishul, M. He, C. You, C. Yu and H. Tan, RSC Adv., 2016, 6, 3840 RSC.
- X. Wang and E. Ruckenstein, Biotechnol. Prog., 1993, 9, 661 CrossRef CAS PubMed; A. Koh, Y. Lu and M. H. Schoenfisch, Anal. Chem., 2013, 85, 10488 CrossRef PubMed; X. Zhang, Z. Du, W. Zou, H. Li, C. Zhang, S. Li and W. Guo, RSC Adv., 2015, 5, 65890 RSC.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS; K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef.
- A. C. Sudik, A. P. Cote, A. G. Wong-Foy, M. O’Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2006, 45, 2528 CrossRef CAS PubMed , and references therein.
- (a) Y. Luan, Y. Qi, H. Gao, R. S. Andriamitantsoa, N. Zheng and G. Wang, J. Mater. Chem. A, 2015, 3, 17320 RSC; (b) M. H. Moemeni, M. A. Amrollahi and F. Tamaddon, Bulg. Chem. Commun., 2015, 47, 7 Search PubMed.
- A. Pande, K. Ganesan, A. K. Jain, P. K. Gupta and R. C. Malhotra, Org. Process Res. Dev., 2005, 9, 133 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: FT-IR spectra, TGA curve, SEM images, EDX spectra and CHNS analysis of MPU and recycled MPU, and characterization of the MPU catalysed Knoevenagel and Henry reaction products by NMR. See DOI: 10.1039/c6cc02578a |
| ‡ Research fellow funded by Alexander von Humboldt Foundation, D-53173 Bonn, Germany. |
| This journal is © The Royal Society of Chemistry 2016 |
