Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbogenically coated silica nanoparticles and their forensic applications†
D.
Fernandes
a,
M. J.
Krysmann
b and
A.
Kelarakis
*a
aCentre for Materials Science, School of Physical Sciences and Computing, University of Central Lancashire, Preston PR12HE, UK. E-mail: akelarakis@uclan.ac.uk; Tel: +44(0) 17724172
bSchool of Pharmacy and Biosciences, University of Central Lancashire, Preston PR12HE, UK
First published on 6th June 2016
Abstract
Carbogenically coated silica nanoparticles (C-SiO2) exhibit color-tunability and carry great promise for two important forensic applications. First, the C-SiO2 nanopowders are ideal for fingerprint development, yielding strong contrast against multicoloured and patterned backgrounds. Second, spontaneous nanoparticle aggregation leads to non-duplicable, inexpensive nanotags that can support sustainable technologies to combat counterfeiting.
The remarkable photophysical properties of carbogenic nanoparticles (otherwise known as C-dots) are systematically explored in a variety of applications including printing inks, photocatalysis, biologically labelling, bioimaging and chemical sensing.1–5 Their non-toxic and biocompatible nature6,7 endows distinct advantages over conventional polyaromatic dyes and heavy metal-based quantum dots.
Large-scale and cost-effective synthesis of C-dots is realised via pyrolytic decomposition of virtually any type of carbon-rich precursors including plants tissue,8 natural saccharides,9,10 animal products11 and synthetic polymers.12,13 Alternatively, C-dots with varying graphitization degree are derived through disintegration,14 hydrothermal treatment15 and electrooxidation16 of carbon nanotubes, carbon nanofibers and graphite. In principle, quantum yield can be improved via surface passivation,17 heteroatom doping18 and the in situ formation of organic chromophores.19,20
In this report we focus on the exploitation of the advanced photophysical properties of carbogenically coated silica nanoparticles (C-SiO2) in two distinct forensic applications. First, we demonstrate that the colour-tuneable C-SiO2 can be used as high performance nanopowders for latent fingerprint enhancement. Second, spontaneous aggregation of the C-SiO2 during solvent evaporation generates non-duplicable photoluminescent motives that are ideal nanotags to mark and authenticate products. As reviewed recently a number of studies report the use of SiO2 based nanomaterials for fingerprint enhancement.21 Notable contributions focus on the use of surface modified SiO2 nanoparticles to gain insights into the mechanisms responsible for fingermark detection22 and the use of fluorescent dye-doped SiO2 nanoparticles to improve the image homogeneity and reduce donor inter-variability.23
To synthesize C-SiO2, colloidal silica was treated with dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride to give a product with 34 wt% organic content as determined by TGA (ESI,† Fig. S1). Subsequently, the product was pyrolysed at 250 °C before being subjected to surface oxidation via HNO3, amine functionalization and excessive dialysis against water. Elemental analysis of C-SiO2 thus received suggests the presence of 26% C, along with minor amounts of H and N (4 and 5%, respectively). The FTIR spectrum (ESI,† Fig. S2) shows peaks attributed24,25 to the vibrational stretching of C–H (1380 cm−1), the anti-symmetric and symmetric stretching vibrations of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1550 cm−1 and 1658 cm−1, respectively) and symmetric (2850 cm−1) and anti-symmetric (2920 cm−1) stretching vibration of sp2 C–H.
O (1550 cm−1 and 1658 cm−1, respectively) and symmetric (2850 cm−1) and anti-symmetric (2920 cm−1) stretching vibration of sp2 C–H.
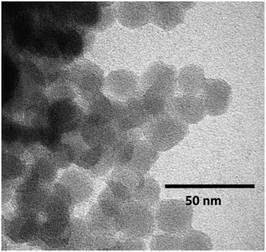
The TEM image of C-SiO2 (Fig. 1) indicates the presence of spherical nanoparticles with average diameter 22 ± 2 nm. The nanoparticles are easily dispersed in water forming stable colloidal dispersions with pH = 10.2, consistent with the presence of surface functionalities generated through the acid/amine treatment. Fig. 2 suggests that the aqueous dispersions exhibit excitation wavelength dependent photoluminescent emission, in a manner similar to that observed for other carbon-based nanoemitters such as C-dots and graphene dots. The emission mechanism is not thoroughly understood, however contributions stemming from surface defects and the conjugated π-domains have been identified.26,27
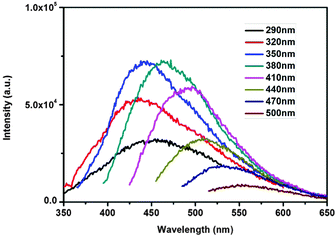 | ||
| Fig. 2 Photoluminescence spectra of C-SiO2 aqueous solutions under different excitation wavelengths. | ||
The freeze-dried fine powder shows excellent flowability and can be easily applied to a variety of non-porous surfaces (ESI,† Fig. S3). The nanopowder adheres strongly to the fingerprints revealing well-resolved patterns that meet the standard for individual identification (ESI,† Fig. S3 and S4). Automated Fingerprint Identification System (AFIS) analysis of a fingerprint developed by C-SiO2 reveals 73 minutiae (details of a fingerprint such as ridge ending, ridge bifurcation, etc) compared to 65 minutiae for an identical fingerprint developed by a standard white fingerprint powder (WFP) (ESI,† Fig. S5).
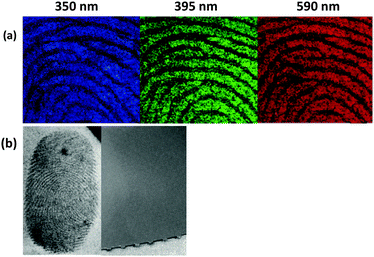
Significantly, as shown in Fig. 3a the nanopowders exhibit colour-tunability and emit within the blue, green and red region when they are irradiated by violet, blue and green light, respectively. The supreme photophysical properties of C-SiO2 can open new horizons in forensic investigation by bypassing problems related to strong background interference from overlapping letters or fluorescent, multi-coloured and textured substrates. To demonstrate this behaviour, a set of fingerprints deposited on a highly fluorescent cardboard were developed under identical conditions using the C-SiO2 nanopowder and a commercial WFP, respectively. High quality images are collected for the C-SiO2 enhanced fingerprints (left image in Fig. 3b) under a 445 nm wavelength laser on a crime-lite imager. In contrast, the fingerprints developed using the standard WFP suffer from strong background interference leading to poor image quality (right image in Fig. 3b). A full set of images under the different wavelengths available on the crime lite imager can be seen in ESI,† Fig. S6. Moreover, a fingerprint developed with C-SiO2 compares favourably with that developed by a commercial fluorescent powder, in the sense that the latter shows strong contrast only under certain illumination wavelengths (ESI,† Fig. S7).
Fingerprint matching remains by far the most reliable biometric method used by law enforcement for individual identification, but its accuracy critically depends on the quality of the recovered impressions. To that end, a large number of fingerprint powders have been developed to maximize contrast against a variety of radically different substrates. In that sense, the use of a single, yet colour-tunable C-SiO2 nanopowder offers significant advantages in terms of dusting speed and image quality.
We have recently introduced a close related class of nanopowders comprising minor amounts of C-dots that also exhibit colour-tunability with respect to the incident radiation and, thus, are suitable for fingerprint enhancement.28 Another study describes the use of aqueous dispersions of C-dot/poly(dimethylacrylamide) nanocomposites for fingerprint detection.29 Notably, nanopowders with high C-dot content are not photoactive, presumably due to extensive self-quenching effects.30 A recent study suggests that blue, green and tan silicon–carbon dots/silica nanocomposite powders show adjustable optical properties as a function of their composition and structure.31 Here we demonstrate colour-tuneable nanopowders containing a single type of nanoparticles and are, thus, distinctly different from the nanocomposite and hybrid materials reported previously. The main advantage of C-SiO2 nanoparticles compared to C-dots based nanocomposites refers to their much larger carbogenic surface that holds great promise for selective and sensitive sensing of forensically important compounds found in fingerprints. We note that this report is a proof-of-concept investigation corresponding to phase 1 pilot studies as described by the International Fingerprint Research Group.32
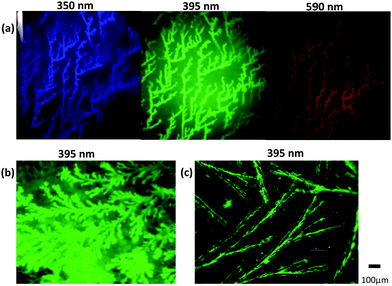
When the pH of the aqueous suspension of C-SiO2 drops below 8.5, the colloidal stability is compromised as evident by the evolution of large aggregates (ESI,† Fig. S8). During water evaporation from an acidified dispersion, the C-SiO2 were seen to undergo spontaneous and random self-assembly, giving rise to motives that are highly fluorescent under laser radiation as shown in Fig. 4a. For comparison, we display two more images; one derived from a more concentrated dispersion (Fig. 4b) and one formed on a polymeric surface (Fig. 4c). (In contrast, no patterns are formed from dispersions with pH = 10, as shown in ESI,† Fig. S9).
The SEM images displayed in Fig. 5 reveal a high level of structural complexity confirming that the motives are nearly impossible to duplicate and can serve as nanotags for object authentication. The nanotags adhere strongly to a variety of substrates (polymers, metals, glass) and remain unaltered even after prolonged exposure to high temperature (ESI,† Fig. S10). We attribute this remarkable structural stability and durability to their organic-free and polymeric-free composition.
Suffice to say that counterfeit accounts for a 5–7% world trade, causing a severe financial drain to the global economy. This crime deprives taxable income from bona fide business, discourages investment for innovation and inhibits social progress. Counterfeit drugs not only fail to cure, but they can cause severe health issues. Recent trends to address this problem rely on the use of upconverting nanocrystals33 and dye-doped nanoparticles34 in integrated safety features and security graphics; however, those sophisticated compounds are often toxic and prohibitively expensive. In contrast, C-SiO2 based nanotags are cost-effective and non-toxic for humans and the environment. Moreover, by virtue of their colour-tuneable nature, C-SiO2 based nanotags impart enhanced security features, compared to commonly used dyes that display only one fixed colour.
In conclusion, we introduce a new class of colour-tuneable nanomaterials (C-SiO2) and we demonstrate their great potential in two distinct forensic applications. As fingerprint powders they circumvent problems stemming from poor image quality against multicoloured substrates. As nanoparticles that undergo spontaneous and random aggregation, they generate non-repeatable motives with a high level of structural complexity that can be used to mark and authenticate products. Those inexpensive and non-toxic nanotags are viable alternatives to current solutions with prohibitively high cost and they can support sustainable technologies to combat counterfeiting.
References
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed
.
- K. Hola, Y. Zhang, Y. Wang, E. P. Giannelis, R. Zboril and A. L. Rogach, Nano Today, 2014, 9, 590–603 CrossRef CAS
.
- A. Kelarakis, MRS Energy Sustainability, 2014, 1, 1–15 CrossRef
.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC
.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2014, 44, 362–381 RSC
.
- H. Tao, K. Yang, Z. Ma, J. Wan, Y. Zhang, Z. Kang and Z. Liu, Small, 2012, 8, 281–290 CrossRef CAS PubMed
.
- J. Wei, J. Shen, X. Zhang, S. Guo, J. Pan, X. Hou, H. Zhang, L. Wang and B. Feng, RSC Adv., 2013, 3, 13119–13122 RSC
.
- M. J. Krysmann, A. Kelarakis and E. P. Giannelis, Green Chem., 2012, 14, 3141–3145 RSC
.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563–5565 CrossRef CAS
.
- L. Zhou, B. He and J. Huang, Chem. Commun., 2013, 49, 8078–8080 RSC
.
- J. Wang, C. F. Wang and S. Chen, Angew. Chem., Int. Ed., 2012, 51, 9297–9301 CrossRef CAS PubMed
.
- J. Gu, W. Wang, Q. Zhang, Z. Meng, X. Jia and K. Xi, RSC Adv., 2013, 3, 15589–15591 RSC
.
- A. Jaiswal, S. S. Ghosh and A. Chattopadhyay, Chem. Commun., 2012, 48, 407–409 RSC
.
- L. Lin and S. Zhang, Chem. Commun., 2012, 48, 10177–10179 RSC
.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed
.
- J. Zhou, C. Booker, R. Li, X. Zhou, T. K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed
.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650–10652 RSC
.
- Y. Dong, H. Pang, H. Bin Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 125, 7954–7958 CrossRef
.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2012, 134, 747–750 CrossRef CAS PubMed
.
- S. Zhu, X. Zhao, Y. Song, S. Lu and B. Yang, Nano Today, 2016, 11, 128–132 CrossRef CAS
.
- A. Leśniewski, Synth. Met., 2016 DOI:10.1016/j.synthmet.2016.03.032
.
- S. Moret, A. Bécue and C. Champod, Nanotechnology, 2014, 25, 1–10 CrossRef PubMed
.
- S. Moret, A. Bécue and C. Champod, Forensic Sci. Int., 2016, 259, 10–18 CrossRef CAS PubMed
.
- X. Cui, L. Zhu, J. Wu, Y. Hou, P. Wang, Z. Wang and M. Yang, Biosens. Bioelectron., 2015, 63, 506–512 CrossRef CAS PubMed
.
- L. Stobinski, B. Lesiak, L. Kover, J. Toth, S. Biniak, G. Trykowski and J. Judek, J. Alloys Compd., 2010, 501, 77–84 CrossRef CAS
.
- A. Kelarakis, Curr. Opin. Colloid Interface Sci., 2015, 20, 354–361 CrossRef CAS
.
- Z. Gan, H. Xu and Y. Hao, Nanoscale, 2016, 8, 7794–7807 RSC
.
- D. Fernandes, M. J. Krysmann and A. Kelarakis, Chem. Commun., 2015, 51, 4902–4905 RSC
.
- J. Dilag, H. Kobus, Y. Yu, C. T. Gibson and A. V. Ellis, Polym. Int., 2015, 64, 884–891 CrossRef CAS
.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed
.
- C. Shih, P. Chen, G. Lin, C. Wang and H. Chang, ACS Nano, 2015, 9, 312–319 CrossRef CAS PubMed
.
- International Fingerprint Research Group (IFRG), J. Forensic Identif., 2014, 64, 174–200 Search PubMed
.
- N. M. Sangeetha, P. Moutet, D. Lagarde, G. Sallen, B. Urbaszek, X. Marie, G. Viau and L. Ressier, Nanoscale, 2013, 5, 9587–9592 RSC
.
- J. Kim, J. M. Yun, J. Jung, H. Song, J.-B. Kim and H. Ihee, Nanotechnology, 2014, 25, 155303 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental section, TGA thermograph, FTIR spectra, AFIS analysis of fingerprints, hydrodynamic diameter and fluorescence images of fingerprints and nanotags. See DOI: 10.1039/c6cc02556k |
| This journal is © The Royal Society of Chemistry 2016 |