Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bis-phosphine allene ligand: coordination chemistry and preliminary applications in catalysis†‡
Avassaya
Vanitcha
a,
Cecilia
Damelincourt
a,
Geoffrey
Gontard
a,
Nicolas
Vanthuyne
b,
Virginie
Mouriès-Mansuy
*a and
Louis
Fensterbank
*a
aSorbonne Universités UPMC Univ Paris 06, CNRS, UMR 8232, Institut Parisien de Chimie Moléculaire, 4 place Jussieu, C.229, F-75005 Paris, France
bAix-Marseille Université, Centrale Marseille, CNRS, iSm2 UMR 7313, 13397, Marseille, France
First published on 12th April 2016
Abstract
A 1,3-bis-diphenylphosphine allene can give rise to new coordination complexes with palladium, platinum and gold metals. These complexes were fully characterized by NMR, HRMS and X-ray diffraction analysis. For gold(I), the corresponding dinuclear complex has been used in a series of diagnostic catalytic reactions and gave promising preliminary results in asymmetric catalysis.
Coordination chemistry has been at the center of chemists' preoccupations since the pioneering works of Christian Wilhem Blomstrand, Sophus Mads Jorgensen and Alfred Werner in the 19th century. This very fundamental and intense domain of investigation has continuously irrigated several fields of applications such as catalysis, material sciences, and supramolecular and medicinal chemistry.1 Based on the interaction between a ligand and a metal center, the game of combining both components appears unlimited and the number of existing coordination adducts is still restricted, leaving a vast unexplored chemical space. Nevertheless, a rational design is desirable to guarantee the preparation of materials with optimized properties. This approach generally requires one to start from elementary building blocks (ligand or metal) with particular attributes.
Thus, thanks to its unique stereoelectronic features, an allene scaffold bearing a Lewis base and possibly conveying some chiral information sites appeared to us as a valuable keystone (Scheme 1).2 As a further incentive, there has been, to the best of our knowledge, a very limited number of allene-derived ligands involved in metallic coordination complexes. Krause reported the formation of silver and copper complexes from allene-containing bipyridine ligands, but no catalytic activity was reported.3 Chiral diphosphine oxide allenes were first used as organocatalysts by Ready who described the highly enantioselective formation of chlorohydrins from meso-epoxides with SiCl4.4 Later, the same group devised chiral allene-containing bisphosphines that, when coordinated to Rh(I), were able to promote the asymmetric addition of arylboronic acids to α-keto esters with high enantioselectivity.5
In line with this strategy, our attention was drawn to the bis-phosphine 1,3-bis(diphenylphosphino)-1,3-diphenylallene (1) featuring direct attachment of the phosphorus moieties on the allene scaffold, which has been so far very rarely encountered.6 The Schmidbaur group previously described the synthesis of 1.7 Following their procedure, we obtained 1 in a consistent yield of 48% (Scheme 2).
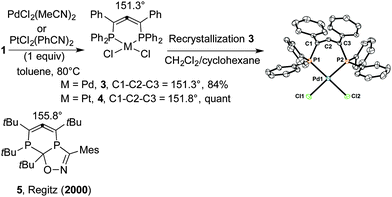
We then studied the unexplored coordination properties of 1 and focused on the possibility of obtaining mononuclear palladium(II) and platinum(II) complexes. Thus, heating at 80 °C for 4 h, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of bis(acetonitrile)dichloropalladium and 1 in toluene afforded the coordination complex 3 (84% yield) that was isolated as an orange solid after precipitation in cold ether. 31P NMR showed a singlet peak resonance at δP = 128.3 ppm, very downfield compared to the starting allene 1 (δP = 33.2 ppm) and the 13C peak of the central allene carbon was observed as a triplet peak (t, 2JCP = 3.8 Hz) at 193.3 ppm (δ = 209.9 ppm, d, 2JCP = 3.5 Hz for 1). An initial crystallization attempt of the bis-P-coordinated Pd allene complex 3 in MeOH provided suitable material for a single-crystal X-ray diffraction (XRD) analysis. Interestingly, the latter showed a co-crystallized mixture of MeOH and HCl adducts on 3 (see ESI‡). While these findings strongly suggested the formation of the Pd–allene complex 3, its structure was fully confirmed after XRD analysis of crystals grown in a CH2Cl2/cyclohexane mixture (Scheme 3).8 A square planar coordination was observed. More striking was the severely bent character of the allene moiety, exhibiting a C
1 mixture of bis(acetonitrile)dichloropalladium and 1 in toluene afforded the coordination complex 3 (84% yield) that was isolated as an orange solid after precipitation in cold ether. 31P NMR showed a singlet peak resonance at δP = 128.3 ppm, very downfield compared to the starting allene 1 (δP = 33.2 ppm) and the 13C peak of the central allene carbon was observed as a triplet peak (t, 2JCP = 3.8 Hz) at 193.3 ppm (δ = 209.9 ppm, d, 2JCP = 3.5 Hz for 1). An initial crystallization attempt of the bis-P-coordinated Pd allene complex 3 in MeOH provided suitable material for a single-crystal X-ray diffraction (XRD) analysis. Interestingly, the latter showed a co-crystallized mixture of MeOH and HCl adducts on 3 (see ESI‡). While these findings strongly suggested the formation of the Pd–allene complex 3, its structure was fully confirmed after XRD analysis of crystals grown in a CH2Cl2/cyclohexane mixture (Scheme 3).8 A square planar coordination was observed. More striking was the severely bent character of the allene moiety, exhibiting a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond angle of 151.2(2)°. The C1–C2 and C2–C3 bond lengths of respectively 1.303(3) and 1.315(3) Å lie in the typical range observed for cumulenes.9
C bond angle of 151.2(2)°. The C1–C2 and C2–C3 bond lengths of respectively 1.303(3) and 1.315(3) Å lie in the typical range observed for cumulenes.9
Similarly, platinum complex 4 was formed quantitatively. 31P NMR shows a central resonance at δP = 98.7 ppm with two small satellite peaks due to 195Pt (1J31P–195Pt = 4371 Hz). Here also, the 13C peak of the allene central carbon is more shielded (δ = 198.9 ppm, 2JCP = 5.1 Hz). The XRD analysis8 confirmed the structure and also showed a square planar coordination with a similar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bent angle of 151.8(4)° (see ESI‡).
C bent angle of 151.8(4)° (see ESI‡).
A few bent allenes have previously been reported10 though there has been some controversy in assigning an allenic character to some of them.11 The most structurally related example to 3 or 4 corresponds to cyclic allene 5, previously isolated by Regitz and exhibiting a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C angle of 155.8°.6c While palladium complex 3 appears quite stable, platinum complex 4 decomposed in a few days upon standing on the bench at room temperature.
C angle of 155.8°.6c While palladium complex 3 appears quite stable, platinum complex 4 decomposed in a few days upon standing on the bench at room temperature.
These preliminary findings validated the coordination ability of allene 1 so we looked at the possible formation of gold complexes in connection with our interest in gold catalysis.12,13 Our initial attempt focused on the formation of a tricoordinate mononuclear complex as more and more examples14 of this coordination mode have been reported and shown intriguing properties.15 Interestingly, when mixing 1.1 equiv. of 1 with 1 equiv. of Me2SAuCl in CH2Cl2 at −20 °C, a new species (δP = 12.5 ppm, broad peak) was selectively formed. The XRD analysis revealed the polymeric gold complex 6.16,17 While the properties of these macromolecular complexes will be studied, we turned our attention to dinuclear complexes.
Thus, treatment of 1 with 2 equiv. of Me2SAuCl in CH2Cl2 provided quantitatively the chiral gold(I) complex rac-7. The latter proved to be quite stable to air and moisture. Its structure was also confirmed by XRD studies (Scheme 4).17 Despite the known carbophilicity of gold(I) salts,18 coordination took place exclusively on the phosphine moieties, leaving the allene unaltered. The two Au atoms lie in opposite direction, with an Au–Au distance of 6.498(1) Å precluding any aurophilic interaction.19 The distance between the Au atom and the phenyl group of the allene moiety is, on average, 3.71(2) Å suggesting no η1 or η2 interaction as described for the JohnPhos gold(I) chloride complex and congeners.20
The evaluation of the electronic properties of allene 1 was achieved by measuring the magnitude of 1JP–Se for the 77Se isotopomer21 of the corresponding bis-selenide derivative 2 (Scheme 2). With a 1JP–Se value of 754 Hz, allene 1 exhibits an intermediate σ-donor ability, lower than that of PPh3 (1JP–Se = 728 Hz) but much higher than that of P(PhO)3 (1JP–Se = 1027 Hz).22
We then evaluated the catalytic activity of gold complex rac-7 using the prototypical N-tethered 1,6-enyne precursor 8a. The reaction was carried out in CH2Cl2 with a catalytic 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of rac-7 (3 mol%) and AgSbF6 to afford diene 9a in 74% yield. When 1,6-enyne 8b was treated under the same conditions, only starting material was recovered even under refluxing conditions. An additional 3 mol% of silver salt gave the expected product 9b in 52% yield. It is noteworthy that when 2 equiv. of AgSbF6 (6 mol%) were introduced right from the start of the reaction, compound 9a was obtained in similar yield as with 3 mol% but we observed a better yield (85%) for compound 9b (Scheme 5).
1 mixture of rac-7 (3 mol%) and AgSbF6 to afford diene 9a in 74% yield. When 1,6-enyne 8b was treated under the same conditions, only starting material was recovered even under refluxing conditions. An additional 3 mol% of silver salt gave the expected product 9b in 52% yield. It is noteworthy that when 2 equiv. of AgSbF6 (6 mol%) were introduced right from the start of the reaction, compound 9a was obtained in similar yield as with 3 mol% but we observed a better yield (85%) for compound 9b (Scheme 5).
As previously reported in the literature23 and by us,24 we suspected the detrimental formation of a chloride-bridged digold complex. The latter was detected by ESI MS at 20 V (see ESI‡) from an equimolar mixture of 1 and AgSbF6 in CH2Cl2, as attested by the presence of a peak at m/z 989 (C39H30Au2ClP2+). This complex would be cleaved by more coordinating substrates like 8a or by the addition of an excess of silver salt yielding a dicationic gold catalytic species.
Following the previously defined reaction conditions, we extended the investigation of the electrophilic catalytic properties of rac-7 by examining the reactivity of several representative polyunsaturated substrates. Thus, enyne 10 provided regioselectively cyclopentadienic dienes 11a,a′25 in 70% yield with no cyclohexadienic product.13c Running the same reaction in methanol as solvent did not affect the catalytic activity and gave smoothly methoxycyclization adduct 12 as a single regioisomer.25 The quantitative conversion of enyne 13 into tricyclic derivative 14 confirmed the robustness of this catalytic system.26
Intermolecular gold-catalyzed additions offer interesting synthetic opportunities. We evaluated the intermolecular cyclopropanation reaction between propargyl pivalate 15 and styrene and were pleased to obtain cyclopropane adduct 16 in 60% yield and with improved diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in favor of the cis-isomer than previously reported (>6
1) in favor of the cis-isomer than previously reported (>6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).27 The cycloisomerization of allenediene 17 served as a very informative probe. It showed that the chemoselective activation of an allene was possible with our allene-based catalytic system since a 4
1).27 The cycloisomerization of allenediene 17 served as a very informative probe. It showed that the chemoselective activation of an allene was possible with our allene-based catalytic system since a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of [4+2] and [4+3] cycloadducts 18 and 19 was obtained in 67% yield. The major formation of 18 suggests a rather electron-depleted catalytic species, more electrophilic than with triphenylphosphine as ligand which gives a 2
1 mixture of [4+2] and [4+3] cycloadducts 18 and 19 was obtained in 67% yield. The major formation of 18 suggests a rather electron-depleted catalytic species, more electrophilic than with triphenylphosphine as ligand which gives a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 18 and 19.28 This product distribution is thus consistent with our preliminary evaluation of the electronics with bis-seleno derivative 2 (Scheme 6).
1 ratio of 18 and 19.28 This product distribution is thus consistent with our preliminary evaluation of the electronics with bis-seleno derivative 2 (Scheme 6).
We finally wished to look at the possibility of using the chirality of ligand 1 for asymmetric transformations.29 Although the number of optical resolutions of organometallic complexes by preparative HPLC remains limited,30 and to the best of our knowledge none with gold,31 we tried this method on the gold chloride complex rac-7. Gratifyingly, the enantiomers of complex rac-7 were nicely separated by analytical chiral chromatography on a Chiralpak IE column. Each enantiomer was obtained with high ee of 98% and their absolute configuration determined by anomalous XRD.32 We then evaluated the catalytic activity of (aR)-(+)-7 for the asymmetric cycloisomerisation of 1,6-enynes 8b and 20. The expected azabicyclo[4.1.0]heptenes 9b and 21 were obtained in good yields and with promising ee values of 29 and 32%, respectively (Scheme 7).
This journey in the coordination of bis-phosphine allene 1 with palladium, platinum and gold salts has allowed the formation of highly novel and original coordination complexes. Physico-chemical properties of these complexes will be pursued. Focusing on gold(I) salts, we could generate a new type of chiral dinuclear gold precatalyst. The precatalyst rac-7 in the presence of a silver salt proved to be very active and robust in a series of prototypical gold-catalyzed reactions. Investigation of the electronic properties suggests a moderately electrophilic gold complex which was corroborated by catalytic results. Preliminary testing of the corresponding optically pure complex obtained by preparative chiral HPLC gave promising hits for asymmetric catalysis. It is anticipated that fine electronic and steric tuning of the allene bis-phosphine ligand by appropriate substitution should boost the catalytic properties.
Notes and references
- (a) Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Boston, 2004 Search PubMed; (b) Comprehensive Organometallic Chemistry, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier, 3rd edn, 2007 Search PubMed; (c) Bioorganometallic Chemistry: Applications in Drug Discovery, Biocatalysis and Imaging, ed. G. Jaouen and M. Salmain, Wiley, 2015 Search PubMed; (d) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848 RSC; (e) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728 RSC; (f) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703 RSC; (g) T. R. Cook, Y. R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed; (h) D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 CrossRef CAS; (i) C. G. Hartinger, N. Metzler-Nolte and P. J. Dyson, Organometallics, 2012, 31, 5677 CrossRef CAS.
- (a) Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 CrossRef CAS PubMed.
- S. Löhr, J. Averbeck, M. Schürmann and N. Krause, Eur. J. Inorg. Chem., 2008, 552 CrossRef.
- X. Pu, X. Qi and J. M. Ready, J. Am. Chem. Soc., 2009, 131, 10364 CrossRef CAS PubMed.
- F. Cai, X. Pu, X. Qi, V. Lynch, A. Radha and J. M. Ready, J. Am. Chem. Soc., 2011, 133, 18066 CrossRef CAS PubMed.
- (a) H. Schmidbaur and T. Pollock, Angew. Chem., Int. Ed., 1986, 25, 348 CrossRef; (b) H. Schmidbaur, T. Pollock, G. Reber and G. Müller, Chem. Ber., 1987, 120, 1403 CrossRef CAS; (c) M. A. Hofmann, U. Bergsträßer, G. J. Reiß, L. Nyulászi and M. Regitz, Angew. Chem., Int. Ed., 2000, 39, 1261 CrossRef CAS; (d) G. Gangadhararao, R. N. P. Tulichula and C. K. Swamy, Chem. Commun., 2015, 51, 7168 RSC.
- H. Schmidbaur, C. M. Frazão, G. Reber and G. Müller, Chem. Ber., 1989, 122, 259 CrossRef CAS.
- CCDC 1448365 (3) and 1448367 (4).
- R. P. Johnson, Chem. Rev., 1989, 89, 1111 CrossRef CAS.
-
(a) D. S. Patel and P. V. Bharatam, J. Org. Chem., 2011, 76, 2558 CrossRef CAS PubMed;
(b) E. Weber, W. Seichter, B. Hess, G. Will and H. J. Dasting, J. Phys. Org. Chem., 1995, 8, 94 CrossRef CAS;
(c) see also in ref. 5: a Pt(allenyl monophosphine) complex showed a severely bent allene (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C angle of 152°) due to a Pt–allene interaction.
C angle of 152°) due to a Pt–allene interaction. - (a) V. Lavallo, C. A. Dyker, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2009, 48, 1540 CrossRef CAS; (b) M. Christl and B. Engels, Angew. Chem., Int. Ed., 2009, 48, 1538 CrossRef CAS PubMed.
- For recent reviews, see: (a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS PubMed; (b) M. Jia and M. Bandini, ACS Catal., 2015, 5, 1638 CrossRef CAS; (c) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC; (d) See also a special Gold Catalysis issue of Accounts of Chemical Research: C. Friend and A. S. K. Hashmi, Gold Catalysis, Acc. Chem. Res., 2014, 47(3) CrossRef CAS PubMed; (e) D. Pflästerer and A. S. K. Hashmi, Chem. Soc. Rev., 2016, 45, 1331 RSC.
- (a) D. Leboeuf, A. Simonneau, C. Aubert, M. Malacria, V. Gandon and L. Fensterbank, Angew. Chem., Int. Ed., 2011, 50, 6868 CrossRef CAS PubMed; (b) A. Simonneau, F. Jaroschik, D. Lesage, M. Karanik, R. Guillot, M. Malacria, J.-C. Tabet, J.-P. Goddard, L. Fensterbank, V. Gandon and Y. Gimbert, Chem. Sci., 2011, 2, 2417 RSC; (c) M. Guitet, P. Zhang, F. Marcelo, C. Tugny, J. Jimenez-Barbero, O. Buriez, C. Amatore, V. Mouriès-Mansuy, J.-P. Goddard, L. Fensterbank, Y. Zhang, S. Roland, M. Ménand and M. Sollogoub, Angew. Chem., Int. Ed., 2013, 52, 7213 CrossRef CAS PubMed; (d) F. Nzulu, A. Bontemps, J. Robert, M. Barbazanges, L. Fensterbank, J.-P. Goddard, M. Malacria, C. Ollivier, M. Petit, J. Rieger and F. Stoffelbach, Macromolecules, 2014, 47, 6652 CrossRef CAS; (e) A. Simonneau, Y. Harrak, L. Jeanne-Julien, G. Lemière, V. Mouriès-Mansuy, J.-P. Goddard, M. Malacria and L. Fensterbank, ChemCatChem, 2013, 5, 1096 CrossRef CAS; (f) L. Ferrand, N. Das Neves, M. Malacria, V. Mouriès-Mansuy, C. Ollivier and L. Fensterbank, J. Organomet. Chem., 2015, 795, 53 CrossRef CAS; (g) A. Vanitcha, G. Gontard, N. Vanthuyne, E. Derat, V. Mouriès-Mansuy and L. Fensterbank, Adv. Synth. Catal., 2015, 357, 2213 CrossRef CAS.
- (a) O. Crespo, M. C. Gimeno, A. Laguna and P. G. Jones, J. Chem. Soc., Dalton Trans., 1992, 1601 RSC; (b) M. C. Gimeno and A. Laguna, Chem. Rev., 1997, 97, 512 CrossRef.
- (a) For a review, see: M. Joost, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2015, 54, 15022 CrossRef CAS PubMed; (b) J. G. Guenther, S. Mallet-Ladeira, L. Estevez, K. Miqueu, A. Amgoune and D. Bourissou, J. Am. Chem. Soc., 2014, 136, 1778 CrossRef CAS PubMed; (c) H. Yang and F. P. Gabbai, J. Am. Chem. Soc., 2015, 137, 13425 CrossRef CAS PubMed.
- For Au(I)-based polymeric chains, see: (a) S. H. Lim, M. L. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 10229 CrossRef CAS PubMed; (b) V. J. Catalano, M. A. Malwitz, S. H. Horner and J. Vasquez, Inorg. Chem., 2003, 42, 2141 CrossRef CAS PubMed; (c) M. Streitberger, A. Schmied and E. Hey-Hawkins, Inorg. Chem., 2014, 53, 6794 CrossRef CAS PubMed; (d) L.-T. Phang, T. S. A. Hor, Z.-Y. Zhou and T. C. W. Mak, J. Organomet. Chem., 1994, 469, 253 CrossRef CAS; (e) C. Khin, A. S. K. Hashmi and F. Rominger, Eur. J. Inorg. Chem., 2010, 1063 CrossRef CAS.
- CCDC 1448368 (6) and 1448369 (rac-7).
- For reviews on the coordination of gold salts onto allenes: (a) M. Malacria, L. Fensterbank and V. Gandon, Top. Curr. Chem., 2011, 302, 157 CrossRef CAS PubMed; (b) E. Soriano and I. Fernandez, Chem. Soc. Rev., 2014, 43, 3041 RSC; (c) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941 RSC ; For coordination complexes, see: ; (d) A. Fürstner, M. Alcarazo, R. Goddard and C. W. Lehmann, Angew. Chem., Int. Ed., 2008, 47, 3210 CrossRef PubMed; (e) T. J. Brown, A. Sugie, M. G. Leed and R. A. Widenhoefer, Chem. – Eur. J., 2012, 22, 6959 CrossRef PubMed ; for the first description of gold-catalyzed additions to allenes, see: ; (f) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (b) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed.
- P. Pérez-Galán, N. Delpont, E. Herrero-Gómez, F. Maseras and A. M. Echavarren, Chem. – Eur. J., 2010, 16, 5324 CrossRef PubMed.
- (a) D. W. Allen, L. A. March and I. W. Nowell, J. Chem. Soc., Dalton Trans., 1984, 483 RSC; (b) D. W. Allen and B. F. Taylor, J. Chem. Soc., Dalton Trans., 1982, 51 RSC; (c) A. Muller, S. Otto and A. Roodt, Dalton Trans., 2008, 650 RSC; (d) M. R. Axet, M. Barbazanges, M. Augé, C. Desmarets, J. Moussa, C. Ollivier, C. Aubert, L. Fensterbank, V. Gandon, M. Malacria, L.-M. Chamoreau and H. Amouri, Organometallics, 2010, 29, 6636 CrossRef CAS.
- C. Glidewell and E. J. Leslie, J. Chem. Soc., Dalton Trans., 1977, 527 RSC.
- (a) A. Bayler, A. Bauer and H. Schmidbaur, Chem. Ber., 1997, 130, 115 CrossRef CAS; (b) A. Hamel, N. W. Mitzel and H. Schmidbaur, J. Am. Chem. Soc., 2001, 123, 5106 CrossRef CAS PubMed; (c) Y. Zhu, C. S. Day, L. Zhang, K. J. Hauser and A. C. Jones, Chem. – Eur. J., 2013, 19, 12264 CrossRef CAS PubMed; (d) A. Homs, I. Escofet and A. M. Echavarren, Org. Lett., 2013, 15, 5782 CrossRef CAS PubMed; (e) Z. Lu, J. Han, G. B. Hammond and B. Xu, Org. Lett., 2015, 17, 4534 CrossRef CAS PubMed.
- F. Schröder, C. Tugny, E. Salanouve, H. Clavier, L. Giordano, D. Moraleda, Y. Gimbert, V. Mouriès-Mansuy, J.-P. Goddard and L. Fensterbank, Organometallics, 2014, 33, 4051 CrossRef.
- (a) C. Nieto-Oberhuber, S. López and A. M. Echavarren, J. Am. Chem. Soc., 2005, 127, 6178 CrossRef CAS PubMed; (b) C. Bartolomé, Z. Ramiro, D. García-Cuadrado, P. Pérez-Galán, M. Raducan, C. Bour, A. M. Echavarren and P. Espinet, Organometallics, 2010, 29, 951 CrossRef; (c) M. Raducan, M. Moreno, C. Bour and A. M. Echavarren, Chem. Commun., 2012, 48, 52 RSC; (d) S. Ito, S. Kusano, N. Morita, K. Mikami and M. Yoshifuji, J. Organomet. Chem., 2010, 695, 291 CrossRef CAS; (e) M. Raducan, C. Rodríguez-Escrich, X. C. Cambeiro, E. C. Escudero-Adán, M. A. Pericás and A. M. Echavarren, Chem. Commun., 2011, 47, 4893 RSC.
- C. Nieto-Oberhuber, S. López and A. M. Echavarren, J. Am. Chem. Soc., 2005, 127, 6178 CrossRef CAS PubMed.
- M. J. Johansson, D. J. Gorin, S. T. Staben and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 18002 CrossRef CAS PubMed.
- (a) P. Mauleón, R. M. Zeldin, A. Z. González and D. Toste, J. Am. Chem. Soc., 2009, 131, 6348 CrossRef PubMed; (b) I. Alonso, B. Trillo, F. López, S. Montserrat, G. Ujaque, L. Castedo, A. Lledós and J. L. Mascarenas, J. Am. Chem. Soc., 2009, 131, 13020 CrossRef CAS PubMed; (c) D. Benitez, E. Tkatchouk, A. Z. Gonzalez, W. A. Goddard III and F. D. Toste, Org. Lett., 2009, 11, 4798 CrossRef CAS PubMed; (d) M. Alcarazo, T. Stork, A. Anoop, W. Thiel and A. Fürstner, Angew. Chem., Int. Ed., 2010, 49, 2542 CrossRef CAS PubMed.
- (a) A. Marinetti, H. Jullien and A. Voituriez, Chem. Soc. Rev., 2012, 41, 4884 RSC; (b) A. Pradal, P. Y. Toullec and V. Michelet, Synthesis, 2011, 1150 Search PubMed; (c) F. Lopez and J. L. Mascarenas, Beilstein J. Org. Chem., 2013, 9, 2250 CrossRef PubMed; (d) W. Zi and F. D. Toste, Chem. Soc. Rev., 2016 10.1039/c5cs00929d.
- (a) K. Schlögl and M. Widhalm, Chem. Ber., 1982, 115, 3042 CrossRef; (b) L. Norel, M. Rudolph, N. Vanthuyne, J. A. G. Williams, C. Lescop, C. Roussel, J. Autschbach, J. Crassous and R. Reau, Angew. Chem., Int. Ed., 2010, 49, 99 CrossRef CAS PubMed; (c) V. A. Soloshonok, T. Ono, H. Ueki, N. Vanthuyne, T. S. Balaban, J. Burck, H. Fliegl, W. Klopper, J.-V. Naubron, T. T. T. Bui, A. F. Drake and C. Roussel, J. Am. Chem. Soc., 2010, 132, 10477 CrossRef CAS PubMed; (d) T. Tozawa, Y. Komoda, J. Ohnishi, Y. Matsuda, J. Taniuchi, C. Nakanishi and K. Okamoto, US pat., 5298642, 1994 Search PubMed; (e) C. Shen, E. Anger, M. Srebro, N. Vanthuyne, L. Toupet, C. Roussel, J. Autschbach, R. Reau and J. Crassous, Chem. – Eur. J., 2013, 19, 16722 CrossRef CAS PubMed.
- (a) H. Liu, D. Leow, K.-W. Huang and C.-H. Tan, J. Am. Chem. Soc., 2009, 131, 7212 CrossRef CAS PubMed; (b) S. Knoppe, I. Dolamic, A. Dass and T. Bürgi, Angew. Chem., Int. Ed., 2012, 51, 7589 CrossRef CAS PubMed.
- CCDC 1448370 ((aR)-(+)-7) & 1448371 ((aS)-(−)-7).
Footnotes |
| † Dedicated to Antonio Echavarren. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1448365 and 1448367–1448371. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc02316a |
| This journal is © The Royal Society of Chemistry 2016 |