Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Luminescence of a binuclear europium complex bearing a 4-nitrophenolate chromophore: a different way of seeing pH dependence†
Octavia A.
Blackburn
a,
Manuel
Tropiano
a,
Louise S.
Natrajan
b,
Alan M.
Kenwright
c and
Stephen
Faulkner
*a
aChemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. E-mail: Stephen.Faulkner@keble.ox.ac.uk
bSchool of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK
cDepartment of Chemistry, University of Durham, South Road, Durham DH1 3LE, UK
First published on 5th April 2016
Abstract
A europium complex derived from NP-(DO3A)2 exhibits pH-dependent europium-centred luminescence following excitation of the nitrophenolate chromophore. Such behaviour is not observed with an analogous mononuclear complex, suggesting coordination of both lanthanide ions to the phenolate oxygen in the emissive species.
Lanthanide luminescence has proved to be an invaluable tool in bioassays over more than three decades, not least because the long-lived luminescence associated with lanthanide-centred emission can readily be separated from background signals arising from scattered light and autofluorescence using time-gating techniques. When sensitising chromophores are used to circumvent the inherently low probability of f–f transitions in absorption, very low (sub-fM) detection limits can be achieved in assays. Such approaches have since been extended to luminescence imaging microscopy, and exploited in imaging changes in a wide variety of biological analytes.1
Sensitised luminescence generally occurs through the chromophore-centred triplet state, which consequently must be higher in energy than the lanthanide emissive state. This restricts the range of chromophores that can be used to sensitise lanthanide ions that emit in the visible region of the spectrum. Attempts to circumvent this problem have involved using multi-photon excitation to excite relatively simple antenna chromophores,2 changing the lanthanide ion so that near-IR emission is observed following visible light excitation,3 or using antenna chromophores with small singlet–triplet energy gaps to maximise the potential excitation wavelength.4 The first two approaches require specialised equipment, while the third has been restricted in scope by the relatively limited number of suitable chromophores.
The pathways involved in sensitisation can be exploited to give rise to pH dependent changes in luminescence, for instance by changing the energy transfer mechanism5 or by introducing new electron transfer pathways for non-radiative quenching of the lanthanide emissive state.6 Alternatively, changes to the coordination sphere of the lanthanide ion can be induced by deprotonation of neighbouring carboxylate or sulfonamide groups so that they can act as chelating7 or bridging8 ligands only in well-defined pH ranges.
For a number of years, we have been exploring the properties of kinetically stable binuclear and bimetallic lanthanide complexes, and using them to prepare more complicated molecular architectures that exploit their potential as building blocks in covalently linked and self-assembling architectures. Their stability can be remarkable, with complexes remaining intact even under the forcing conditions of diazotisation.9
We now report the synthesis and spectroscopic properties of Eu2.1, which contains two lanthanide-binding domains linked by a 2,6-dimethyl-4-nitrophenolate unit. This complex was found to exhibit remarkable luminescence properties, which we now report in detail. The analogous mononuclear complex, Eu.2, was also synthesised and studied for comparison.
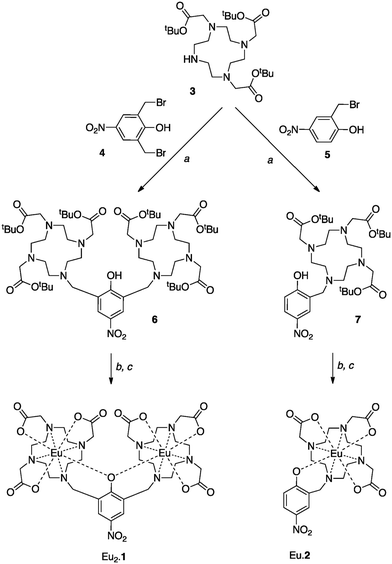
The compounds Eu2.1 and Eu.2 were prepared from the well-known triester, 3,10 as shown in Scheme 1. 2,6-Bis(bromomethyl)-4-nitrophenol (4) was synthesised by a literature procedure11 and reacted with 3 to yield the tert-butyl protected ligand, NP-(DO3A)2, 6; an analogous reaction provided NP-DO3A, 7. Removal of the tert-butyl groups in acidic media and subsequent complexation with europium(III) trifluoromethanesulfonate in water gave Eu2.1 and Eu.2.
To our surprise, we observed that Eu2.1 exhibits luminescence from the europium centres upon both UV excitation and upon excitation with visible light at 405 nm. 4-Nitrophenolates have long been known to be useful and effective chromophores; for instance 4-nitrophenyl esters have been widely used to follow the progress of reactions that involve the formation of 4-nitrophenolate.12 However, they have very low fluorescence quantum yields as a consequence of efficient formation of the triplet excited state from the S1 state. Nevertheless, 4-nitrophenolates have not (to our knowledge) previously been used to sensitise formation of lanthanide-centred excited states. With this in mind, we resolved to study the photophysical properties of the complexes Eu2.1 and Eu.2 in more detail.
The absorption spectrum of Eu2.1 varies dramatically with pH, extending to longer wavelength as the pH is reduced, with λmax shifting from around 340 nm at high/neutral pH to around 380 nm at low pH (Fig. 1a), although it is clear that more than one transition contributes to this region of the spectra, especially at high pH. This contrasts with the behaviour of 4-nitrophenol itself, in which λmax shifts from around 400 nm at high pH to around 320 nm at low pH once the 4-nitrophenolate is completely protonated. In earlier studies on phenol bridged binuclear complexes, we have observed behaviour consistent with the co-existence of a number of isomers. Noting that an ytterbium complex closely related to Eu2.1 exhibits behaviour consistent with the presence of two distinct lanthanide environments on the luminescence timescale (here microseconds),13 we can begin to draw some conclusions about the structural behaviour of the complex in solution.
At low pH, it would be expected that the more open conformers (e.g. B, C in Scheme 2) where the nitrophenolate may be protonated would play a more significant role. The absorption spectra in Fig. 1 can be taken to suggest that C exists in negligible quantities at higher pH, and is the minor component of the mixture even at pH 3. More surprisingly, the major component at low pH is a phenolate species with absorption around 380 nm. We can expect that coordination of two lanthanide ions to the phenolate oxygen will reduce the wavelength at which the absorption maximum is observed, suggesting that A dominates at high pH, while B is the major isomer at pH 3.
Excitation and emission spectra for complex Eu2.1 are shown in Fig. 1, while key data is tabulated in Table 1. Excitation across a broad range of wavelengths, extending into the visible region, resulted in observation of europium-centred emission. Furthermore, while the intensity of the emission varied with pH, the form of the spectrum remains unchanged over a broad pH range (Fig. S1, ESI†). The form of the observed excitation spectrum is also unchanged across the whole pH range studied, and closely resembles the absorption spectrum that we assigned to species A (Fig. S3, ESI†). These observations suggest the presence of a single emissive species, in which the two lanthanide ions are bridged by the phenolate oxygen atom. The luminescence lifetimes measured at different pH values give low q values of ≤0.2 (Table 1) and are within error of one another, consistent with a single emissive species. The relative lack of hydration at the metal centres of the emissive complex would be consistent with structure A where the conformational constraints imposed by phenol coordination force a closed structure where close approach of solvent is restricted.
| pH | λ max/nm | λ exc/nm | τ D2O/ms | τ H2O/ms | q |
|---|---|---|---|---|---|
| 3 | 380 | 334 | 0.52 | 0.49 | 0 |
| 8 | 342 | 334 | 0.82 | 0.61 | 0.2 |
| 12 | 345 | 334 | 0.80 | 0.63 | 0.1 |
To probe this further, we also studied Eu.2, a mononuclear analogue of Eu2.1. The absorption spectrum of Eu.2 behaves similarly to 4-nitrophenol with changing pH (Fig. S6 and S7, ESI†) with the appearance of a higher energy band on lowering the pH, although the pKa of the phenol is clearly affected by the lanthanide containing substituent. In this complex, the uncoordinated phenolate appears to dominate even at low pH. This complex showed no sensitised emission across a broad pH range, implying that coordination sensitised emission is not observed in cases where only one lanthanide is able to coordinate to the phenolate. The lack of observed europium-centred emission from Eu.2 concurs with a previous study of this complex by Sherry and co-workers who postulated that the emissive state was rapidly quenched by a ligand to metal charge transfer (LMCT) state.14 Triplet energy measurements on Gd.2 and Gd2.1‡ (Fig. S4 and S8, ESI†) revealed triplet energies of around 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 for Gd2.1, and 18
500 cm−1 for Gd2.1, and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 cm−1 for Gd.2. However, the complex speciation observed in these systems means that these values need to be treated with caution, though it is clear that sensitised luminescence is thermodynamically feasible in both cases.
100 cm−1 for Gd.2. However, the complex speciation observed in these systems means that these values need to be treated with caution, though it is clear that sensitised luminescence is thermodynamically feasible in both cases.
Our hypothesis as to the nature of the luminescent Eu2.1 species was borne out by an NMR study. The 1H NMR spectra of Eu.2 across a broad pH range (Fig. 2b) are characterised by broad peaks which change position at high pH, which would be consistent with fast exchange between diastereomeric forms of a seven-coordinate complex on the timescale of the NMR experiment.
 | ||
| Fig. 2 1H NMR spectra of (a) Eu2.1 and (b) Eu.2 in D2O (298 K, 400 MHz) at pH 3 (blue), 7 (red) and 12 (black). | ||
The 1H NMR spectra of Eu2.1 at varying pH (Fig. 2a) offer an immediate contrast; the spectra are dominated by two sets of sharp resonances (possibly more) whose positions are pH independent. At low pH, a minor isomer is also observed in which broader lines are seen at similar positions to those of Eu.2, consistent with a heptadentate species. A different set of minor peaks is observed at higher pH; these are sharp, possibly corresponding to coordination of hydroxide at the lanthanide centres. It is clear that the major species in all cases is in slow (or zero) exchange with the visible minor isomer.
Taken together, these results lend weight to the hypothesis that the emissive species in Eu2.1 is one in which both lanthanides are coordinated to the metal centre. It seems clear that the Lewis acidity of both centres is required if we are to modulate the chromophore excited state to the point where it becomes a useful sensitiser.
In conclusion, this work shows the importance of structure and isomerism in controlling lanthanide luminescence behaviour. We believe this to be a highly unusual system in that the photophysical properties of the binuclear complex differ dramatically from those of the mononuclear analogue. This study adds weight to the growing body of evidence that the structure of the whole complex must be taken into account when considering design and function. Furthermore, the potential to use lanthanides themselves to tune the properties of an antenna chromophore opens up a range of new prospects for sensing and control of luminescence properties. In this case, we are exploring the use of this system as both a sensitiser and a controllable luminescent tag.
Notes and references
-
(a) S. Faulkner, L. S. Natrajan, W. S. Perry and D. Sykes, Dalton Trans., 2009, 3890–3899 RSC
; (b) J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef PubMed
; (c) C. P. Montgomery, B. S. Murray, E. J. New, R. Pal and D. Parker, Acc. Chem. Res., 2009, 42, 925–937 CrossRef CAS PubMed
; (d) J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC
.
-
(a) L.-O. Pålsson, R. Pal, B. S. Murray, D. Parker and A. Beeby, Dalton Trans., 2007, 5726–5734 RSC
; (b) G. Piszczek, B. P. Maliwal, I. Gryczynski, J. Dattelbaum and J. R. Lakowicz, J. Fluoresc., 2001, 11, 101–107 CrossRef CAS
; (c) S. V. Eliseeva, G. Auböck, F. van Mourik, A. Cannizzo, B. Song, E. Deiters, A.-S. Chauvin, M. Chergui and J.-C. G. Bünzli, J. Phys. Chem. B, 2010, 114, 2932–2937 CrossRef CAS PubMed
; (d) A. Picot, A. D'Aléo, P. L. Baldeck, A. Grichine, A. Duperray, C. Andraud and O. Maury, J. Am. Chem. Soc., 2008, 130, 1532–1533 CrossRef CAS PubMed
.
-
(a) J.-C. G. Bünzli and S. V. Eliseeva, J. Rare Earths, 2010, 28, 824–842 CrossRef
; (b) N. Shavaleev, S. Pope, Z. R. Bell and S. Faulkner, Dalton Trans., 2003, 808–814 RSC
; (c) A. M. Nonat, C. Allain, S. Faulkner and T. Gunnlaugsson, Inorg. Chem., 2010, 49, 8449–8456 CrossRef CAS PubMed
.
-
(a) A. Dadabhoy, S. Faulkner and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 2000, 2359–2360 RSC
; (b) A. Dadabhoy, S. Faulkner and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 2002, 348–357 RSC
; (c) J. D. Routledge, M. W. Jones, S. Faulkner and M. Tropiano, Inorg. Chem., 2015, 54, 3337–3345 CrossRef CAS PubMed
.
- A. Beeby, S. Faulkner and J. A. G. Williams, J. Chem. Soc., Dalton Trans., 2002, 1918–1922 RSC
.
- D. Parker and J. A. G. Williams, Chem. Commun., 1998, 245–246 RSC
.
- M. P. Lowe, D. Parker, O. Reany, S. Aime, M. Botta, G. Castellano, E. Gianolio and R. Pagliarin, J. Am. Chem. Soc., 2001, 123, 7601–7609 CrossRef CAS PubMed
.
- B. P. Burton-Pye and S. Faulkner, Chem. Commun., 2005, 259–261 Search PubMed
.
- M. P. Placidi, A. J. L. Villaraza, L. S. Natrajan, D. Sykes, A. M. Kenwright and S. Faulkner, J. Am. Chem. Soc., 2009, 131, 9916–9917 CrossRef CAS PubMed
.
- A. Dadabhoy, S. Faulkner and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 2002, 348–357 RSC
.
- J. de Mendoza, P. M. Nieto, P. Prados and C. Sánchez, Tetrahedron, 1990, 46, 671–682 CrossRef CAS
.
- J. Anderson, T. Byrne, K. J. Woelfel, J. E. Meany, G. T. Spyridis and Y. Pocker, J. Chem. Educ., 1994, 71, 715–718 CrossRef CAS
.
- S. J. A. Pope, A. M. Kenwright, S. L. Heath and S. Faulkner, Chem. Commun., 2003, 1550–1551 RSC
.
- M. Woods, G. E. Kiefer, S. Bott, A. Castillo-Muzquiz, C. Eshelbrenner, L. Michaudet, K. McMillan, S. D. K. Mudigunda, D. Ogrin, G. Tircsó, S. Zhang, P. Zhao and A. D. Sherry, J. Am. Chem. Soc., 2004, 126, 9248–9256 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic methods and procedures, additional photophysical spectra. See DOI: 10.1039/c6cc02078j |
| ‡ Obtained by estimating the 0,0 transition in a diethyl ether-ethanol-isopropyl alcohol glass at 77 K. See ESI† for further details. |
| This journal is © The Royal Society of Chemistry 2016 |