Open Access Article
Open Access ArticleA bifunctional chiral [2]catenane based on 1,1′-binaphthyl-phosphates†
R.
Mitra
 a,
M.
Thiele
a,
F.
Octa-Smolin
a,
M. C.
Letzel‡
b and
J.
Niemeyer
*a
a,
M.
Thiele
a,
F.
Octa-Smolin
a,
M. C.
Letzel‡
b and
J.
Niemeyer
*a
aInstitute of Organic Chemistry, Department of Chemistry, University of Duisburg-Essen, Universitätsstrasse 7, 45141 Essen, Germany. E-mail: jochen.niemeyer@uni-due.de
bInstitute of Organic Chemistry, University of Münster, Corrensstrasse 40, 48149 Münster, Germany
First published on 29th March 2016
Abstract
A novel [2]catenane was synthesised by ring-closing metathesis from a Ca-bisphosphate template. The resulting interlocked structure features two chiral 1,1′-binaphthyl-phosphates, leading to a bifunctional catenane structure. Initial binding studies point at the applicability of such mechanically interlocked bisphosphates as artificial receptors for dicationic guest molecules.
Mechanically interlocked molecules (MIMs), such as rotaxanes and catenanes, have inspired chemists for decades.1 Since the initial observations of MIMs as mere lab curiosities, the development of template-based synthetic approaches nowadays allows for their directed synthesis in useful quantities.2 This has set the stage for the use of these topologically fascinating molecules for various applications,3e.g. as molecular shuttles,4 supramolecular catalysts5 and even as nano-sized assembly lines.6
The interlocked nature of rotaxanes and catenanes makes them especially suitable as artificial receptors. The combination of several subunits in a compact interlocked fashion can be used to generate a three-dimensional binding cavity, which leads to strong and specific binding of guest molecules.7 This has been efficiently employed by Beer for the generation of interlocked receptors for various anions, showing that size-selective binding of halides8 and selective binding of dianions over monocharged guests can be achieved.9 The use of interlocked-structures for binding of cationic guest-species has been less systematically studied. Since a pioneering study of Swager on Cu(I)-sensing by a polyrotaxane,10 interlocked sensors for some other metal ions, such as alkali-metal ions have been reported.11 Yashima has recently described a chiral amidinium-carboxylate-based [2]catenane, which acts as a sensor for Zn2+-ions.12
The successful syntheses of chiral mechanically interlocked species,13 even structures lacking any covalent chirality,5a has generated significant interest in their application as chiral host-structures. While Hiratani has reported on the stereoselective sensing of amino alcohols by chiral rotaxanes,14 we believe that the full potential of MIMs as chiral receptors has yet to be fully explored, especially taking into account the highly successful development of non-interlocked species for stereoselective supramolecular sensing.15
Along these lines, we envisaged the synthesis of a new type of chiral bifunctional [2]catenane based on 1,1′-binaphthyl-phosphoric acids. Although these chiral Brønsted-acids have been widely employed in organocatalysis,16 they have not found application in interlocked structures so far. Their integration into a [2]catenane would result in a flexible arrangement of two chiral phosphates in an interlocked molecule, possibly generating useful chiral receptors, especially for the binding of dicationic guest molecules. In this account, we would now like to present the development of a synthetic protocol for the chiral catenane (S,S)-1, which represents the first example of an interlocked structure based on 1,1-binaphthyl-phosphates. Also, we present initial data on its use for the binding of selected chiral dicationic guest molecules.
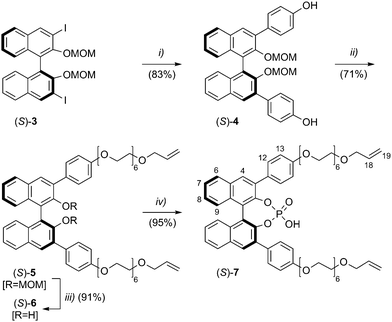
In order to allow for the generation of the desired [2]catenane (S,S)-1 in a double-cyclisation approach, we firstly synthesised the acyclic crescent-shaped BINOL-phosphate (S)-7 (see Fig. 1). The precursor (S)-7 is functionalised with two terminal allyl-substituents which are connected to the 3,3′-positions of the BINOL-backbone via phenyl-hexaethyleneglycol linkers, thus allowing the formation of the macrocyclic subunits of the catenane via ring-closing metathesis (RCM).
The synthesis of (S)-7 was performed in a four-step sequence starting from the previously described MOM-protected diiodide (S)-3.17 Initial introduction of 4-hydroxyphenyl groups in a Suzuki-coupling to give (S)-4 was followed by attachment of the O-allyl-hexaethyleneglycol substituents, resulting in (S)-5.
Removal of the MOM-protecting groups to give (S)-6, followed by an introduction of the phosphate-group yielded the desired BINOL-derivative (S)-7 in 51% overall yield. All compounds were fully characterised by standard spectroscopic and analytical techniques (see ESI†). In addition, chiral HPLC-analysis of compound (S)-6 verified its optical purity (>98% ee).
In order to construct the desired interlocked structure (S,S)-1 starting from the phosphate (S)-7 (see Fig. 2), we envisaged the use of divalent metal ions for the construction of the corresponding intertwined bisphosphate–metal(II) complexes, which would then undergo double RCM. In our hands, calcium(II) was most suitable as a templating agent, so that we decided for the calcium-bisphosphate (S,S)-(8) as the precatenane-structure.
 | ||
| Fig. 2 Synthesis of the catenane (S,S)-1. Reagents and conditions: (i) 0.5 eq. Ca(OMe)2, toluene; (ii) Grubbs-II catalyst, CH2Cl2, purification on RP-18, then washing with HCl. | ||
Compound (S,S)-(8) could easily be obtained by reaction of (S)-7 with Ca(OMe)2. The formation of (S,S)-8 was signalled by shifts in the 1H-NMR (e.g. phenylene-groups: δH = 7.56 and 6.79 ppm for (S,S)-8, cf. δH = 7.61 and 6.92 ppm for (S)-7), accompanied by significant line-broadening, especially in the 1H-NMR (see Fig. 3) and 31P-NMR (δP = 0.5 ppm (ν1/2 = 219 Hz) for (S,S)-8, cf. δP = 1.7 ppm (ν1/2 = 21 Hz) for (S)-7). While complete dissociation of Ca-complex (S,S)-8 was observed in protic solvents (e.g. under conditions of ESI-MS and RP-HPLC, see Fig. S21, ESI†), APCI-MS gave clear indication for the successful synthesis of (S,S)-8 (m/z = 2319.9234, calcd 2319.9237 for [M + H]+, see Fig. S23, ESI†).
 | ||
| Fig. 3 1H-NMR-spectra of the acid (S)-7, the precatenane (S,S)-8, the macrocycle (S)-2 and the catenane (S,S)-1 [for numbering scheme see Fig. 1 and 2, all: 600 MHz, CDCl3, 25 °C]. | ||
For the formation of catenane (S,S)-1, the calcium salt (S,S)-8 was subjected to RCM using Grubbs-II catalyst in diluted dichloromethane solution (1.5 mM). After two days, we observed almost complete consumption of the starting materials and conversion to products of RCM, as judged by the disappearance of the allylic 1H-resonances (δH = 5.82, 5.18 and 5.10 ppm for (S,S)-8) and appearance of new 1H-NMR signals in the area of 5.72–5.64 ppm. Analysis of the reaction mixture by RP-HPLC showed the presence of two main products, which could be purified by reversed phase liquid chromatography.
The firstly eluting product was identified as the undesired non-interlocked macrocycle (S)-2 (22% yield), possibly formed by from (S,S)-8 by backfolding of the flexible ethyleneglycol linkers. (S)-2 was analysed by NMR and ESI-MS (m/z = 1135.44271, calcd 1135.44267 for [(S)-2 + Na]+, see Fig. S44, ESI†) and the assignment of the macrocyclic structure was unambiguously verified by comparison to an independently synthesised sample of (S)-2, which could be generated by RCM from the phosphate (S)-7 (see ESI†).
To our delight, the secondly eluting product could be identified as the desired interlocked catenane (S,S)-1, which we isolated in 14% yield. Positive and negative ESI-MS reveal the catenane (S,S)-1 as the corresponding mono-, di- and triply charged ions with the expected isotopic profiles and accurate masses (e.g. m/z = 2247.89412, calcd 2247.89612 for [(S,S)-1 + Na]+, see Fig. S47–S49, ESI†), none of which were observed for the macrocycle (S)-2.
NMR-spectroscopic analysis of the macrocycle (S)-2 and the catenane (S,S)-1 (see Fig. 3) verifies the formation of chemically different species, most distinctly visible from the different chemical shifts of the phenylene-groups (δ = 7.68, 6.99 ppm for (S)-2 and δ = 7.57, 6.87 ppm for (S,S)-1, cf. 7.56 and 6.79 ppm for (S,S)-8). In the olefinic region, we detect major-signals at δ = 5.65/5.71 ppm, together with minor additional signals at δ = 5.57/5.63 ppm (each for (S)-2/(S,S)-1, see Fig. S25 and S29, ESI†), which we tentatively assign as the (Z)- and (E)-isomers of (S)-2 and the (Z,Z)- and (Z,E)-isomers of (S,S)-1, respectively (ratio major![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) minor = ca. 95
minor = ca. 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in each case). These isomers could not be separated by chromatography so that both (S,S)-1 and (S)-2 were isolated as mixtures of double-bond stereoisomers.
5 in each case). These isomers could not be separated by chromatography so that both (S,S)-1 and (S)-2 were isolated as mixtures of double-bond stereoisomers.
In order to rule out the formation of a non-interlocked [1+1]-macrocycle (see Fig. S41, ESI†), we performed additional investigations by DOSY-NMR and MS/MS-experiments. The hydrodynamic radius of (S,S)-1 was determined as 12.5 Å by DOSY-NMR, which clearly indicates the formation of the interlocked catenane-structure upon comparison to the corresponding calculated values (12.4 Å for (S,S)-1, cf. 16.7 Å for a potential [1+1] macrocycle, for details see ESI†). The interlocked structure of (S,S)-1 was also clearly proven by ESI-MS/MS-measurements. Upon collision-induced dissociation, the catenane (S,S)-1 (mass-selected at m/z = 2248.89 for [(S,S)-1 + Na]+) shows fragmentation to give the macrocycle (S)-2 (m/z = 1135.44322 for [(S)-2 + Na]+) and the corresponding smaller fragments.
Having established the structure of (S,S)-1 as the desired catenane, we tested its applicability as an interlocked receptor. The presence of the two 1,1′-binaphthyl-phosphates should allow for the strong binding of small dicationic guest molecules. We therefore performed a series of NMR-titrations in d6-DMSO using (S,S)-1 as a host for binding of lysine- and arginine methyl esters and trans-1,2-diaminocyclohexane, respectively. In order to obtain information about the stereoselectivity of binding to the chiral catenane, we used both enantiomers of all guest molecules. As a comparison, we also determined the association constants for the macrocycle (S)-2.
Upon addition of the diamine-guests (used as the bis-HCl-salts) to the phosphate hosts (S)-2 and (S,S)-1 (as the mono- or bis-tetrabutylammonium-salts),18 we observe distinct chemical shift changes for the aromatic host signals (see Fig. 4 as a representative example, also see Fig. S53–S64, ESI†). Fig. 5 shows the resulting binding isotherms for the binding of the enantiomeric arginine methyl esters to (S)-2 and (S,S)-1, as determined from the H-8 proton of the hosts (also see Fig. S67–S69, ESI†).19
 | ||
| Fig. 4 Representative stacked plot (aromatic region) of the NMR-titration of (S,S)-1 (as the bis-Bu4N+-salt), with 0.5 to 70 eq. of D-Arg-OMe (as the bis-HCl salt) [for numbering scheme see Fig. 1 and 2, all: 500 MHz, d6-DMSO, 298 K, initial concentration of (S,S)-1: 0.5 mM]. | ||
The binding stoichiometry for binding of the diamines to the catenane (S,S)-1 was established to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as determined from Job-plot analysis (see Fig. S65 and S66, ESI†). For the macrocycle (S)-2, the Job-plot analysis was less conclusive, but comparative data analysis for 1
1, as determined from Job-plot analysis (see Fig. S65 and S66, ESI†). For the macrocycle (S)-2, the Job-plot analysis was less conclusive, but comparative data analysis for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometries indicates a 2
1 stoichiometries indicates a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host
1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complex formation (see ESI† for details). Accordingly, we determined the association constants by analysis of the binding isotherms using 1
guest complex formation (see ESI† for details). Accordingly, we determined the association constants by analysis of the binding isotherms using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding models, respectively. For easier comparison, both Ka values for the 2
1 binding models, respectively. For easier comparison, both Ka values for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding process to (S)-2 were integrated into a single value (see ESI† for details).
1 binding process to (S)-2 were integrated into a single value (see ESI† for details).
The resulting association constants for binding of the diamines to the macrocycle (S)-2 are in the range of 570–870 M−1, indicating a rather weak binding in DMSO-solution (see Table 1). There is little chemoselectivity between the different guest species, and only in the case of the arginine-enantiomers we observe a small degree of stereoselectivity in favour of the D-isomer (Kfav/Kdisfav = 1.3).
| [(S)-2−(Bu4N+)] | [(S,S)12−(Bu4N+)2] | |||
|---|---|---|---|---|
| Diaminea | K a [M−1] | K fav/Kdisfav | K a [M−1] | K fav/Kdisfav |
| a All diamines used as the bis-HCl-salts, all values determined by monitoring the H-8 proton of the host-molecules. b DACH = 1,2-diaminocyclohexane. | ||||
| L-Lys-OMe | 630 ± 20 | 1.1 | 4100 ± 610 | 1.4 |
| D-Lys-OMe | 700 ± 30 | 5700 ± 730 | ||
| L-Arg-OMe | 570 ± 25 | 1.3 | 5550 ± 320 | 1.5 |
| D-Arg-OMe | 770 ± 35 | 8200 ± 610 | ||
| (S,S)-DACHb | 870 ± 170 | 1.0 | 15800 ± 2900 | 1.6 |
| (R,R)-DACHb | 840 ± 100 | 10050 ± 1300 | ||
For the formation of the catenane–diamine complexes however, we find significantly higher association constants of 4100 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1, showing a stronger binding of the guests to the interlocked bisphosphate-receptor (S,S)-1 in comparison to the macrocycle (S)-2. While we observe no significant chemoselectivity between the lysine- and arginine methyl-esters, the larger association constants for the 1,2-diamino-cyclohexanes indicate a stronger binding of this smaller guest-molecule to the catenane-host.
800 M−1, showing a stronger binding of the guests to the interlocked bisphosphate-receptor (S,S)-1 in comparison to the macrocycle (S)-2. While we observe no significant chemoselectivity between the lysine- and arginine methyl-esters, the larger association constants for the 1,2-diamino-cyclohexanes indicate a stronger binding of this smaller guest-molecule to the catenane-host.
More interestingly, we also observe a more significant degree of stereodiscrimination, favouring the binding of the D-amino acids and the (S,S)-enantiomer of 1,2-diaminocyclohexane, respectively. The association constants of the favoured enantiomers are 1.4 to 1.6 times higher than those of the disfavoured ones (see Table 1).
In summary, we have reported on the successful synthesis of a new type of bifunctional chiral [2]catenane based on 1,1′-binaphthyl phosphates. The homo-catenane (S,S)-1 was generated by ring-closing metathesis from a Ca-templated precatenane to generate the desired interlocked structure. Initial binding studies show a strong binding of diamines to the bifunctional catenane, alongside with slight stereospecificity induced by the chiral host structure.
Detailed studies on the variation of the catenane structure and the resulting effects on selective guest binding are currently underway in our laboratories. In addition, we believe that the bifunctional framework of the catenane (S,S)-1 will allow for its use in other applications, e.g. the formation of supramolecular polymers or in supramolecular organocatalysis.
We would like to thank Mr. Manfred Zähres for his help with the DOSY-NMRs and Mr. Martin Ehlers and Mr. Wilhelm Sicking for their help with the Macro-Model calculations. J. N. thanks Prof. Carsten Schmuck for his mentorship and the Fonds der Chemischen Industrie for funding.
Notes and references
- (a) G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, Angew. Chem., Int. Ed., 2015, 54, 6110–6150 CrossRef PubMed; (b) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- (a) G. T. Spence and P. D. Beer, Acc. Chem. Res., 2012, 46, 571–586 CrossRef PubMed; (b) J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260–9327 CrossRef CAS PubMed; (c) J. D. Crowley, S. M. Goldup, A. L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530–1541 RSC; (d) C. Schalley, T. Weilandt, J. Brüggemann and F. Vögtle, in Templates in Chemistry I, ed. C. A. Schalley, F. Vögtle and K. H. Dötz, Springer, Berlin, 2004, ch. 4, vol. 248, pp. 141–200 Search PubMed.
- (a) S. F. M. van Dongen, S. Cantekin, J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, Chem. Soc. Rev., 2014, 43, 99–122 RSC; (b) J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 11102–11104 CrossRef CAS PubMed; (c) E. A. Neal and S. M. Goldup, Chem. Commun., 2014, 50, 5128–5142 RSC; (d) D. A. Leigh, V. Marcos and M. R. Wilson, ACS Catal., 2014, 4, 4490–4497 CrossRef CAS.
- V. Balzani, A. Credi, B. Ferrer, S. Silvi and M. Venturi, Top. Curr. Chem., 2005, 262, 1–27 CrossRef CAS.
- (a) Y. Cakmak, S. Erbas-Cakmak and D. A. Leigh, J. Am. Chem. Soc., 2016, 138, 1749–1751 CrossRef CAS PubMed; (b) M. Galli, J. E. M. Lewis and S. M. Goldup, Angew. Chem., Int. Ed., 2015, 54, 13545–13549 CrossRef CAS PubMed; (c) J. Beswick, V. Blanco, G. De Bo, D. A. Leigh, U. Lewandowska, B. Lewandowski and K. Mishiro, Chem. Sci., 2015, 6, 140–143 RSC; (d) C. B. Caputo, K. Zhu, V. N. Vukotic, S. J. Loeb and D. W. Stephan, Angew. Chem., Int. Ed., 2013, 52, 960–963 CrossRef CAS PubMed; (e) V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, Angew. Chem., Int. Ed., 2012, 51, 5166–5169 CrossRef CAS PubMed; (f) N. Miyagawa, M. Watanabe, T. Matsuyama, Y. Koyama, T. Moriuchi, T. Hirao, Y. Furusho and T. Takata, Chem. Commun., 2010, 46, 1920–1922 RSC; (g) Y. Tachibana, N. Kihara and T. Takata, J. Am. Chem. Soc., 2004, 126, 3438–3439 CrossRef CAS PubMed.
- (a) G. De Bo, S. Kuschel, D. A. Leigh, B. Lewandowski, M. Papmeyer and J. W. Ward, J. Am. Chem. Soc., 2014, 136, 5811–5814 CrossRef CAS PubMed; (b) B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D’Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189–193 CrossRef CAS PubMed.
- (a) M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935–1949 CrossRef CAS PubMed; (b) A. Caballero, F. Zapata and P. D. Beer, Coord. Chem. Rev., 2013, 257, 2434–2455 CrossRef CAS; (c) M. J. Chmielewski, J. J. Davis and P. D. Beer, Org. Biomol. Chem., 2009, 7, 415–424 RSC.
- (a) S. P. Cornes, C. H. Davies, D. Blyghton, M. R. Sambrook and P. D. Beer, Org. Biomol. Chem., 2015, 13, 2582–2587 RSC; (b) S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. L. Thompson, P. Kennepohl and P. D. Beer, J. Am. Chem. Soc., 2014, 137, 499–507 CrossRef PubMed.
- M. J. Langton and P. D. Beer, Chem. – Eur. J., 2012, 18, 14406–14412 CrossRef CAS PubMed.
- S. S. Zhu and T. M. Swager, J. Am. Chem. Soc., 1997, 119, 12568–12577 CrossRef CAS.
- (a) S.-Y. Hsueh, C.-C. Lai and S.-H. Chiu, Chem. – Eur. J., 2010, 16, 2997–3000 CrossRef CAS PubMed; (b) K. Hiratani, M. Kaneyama, Y. Nagawa, E. Koyama and M. Kanesato, J. Am. Chem. Soc., 2004, 126, 13568–13569 CrossRef CAS PubMed; (c) Y. Nagawa, J.-I. Suga, K. Hiratani, E. Koyama and M. Kanesato, Chem. Commun., 2005, 749–751 RSC.
- Y. Nakatani, Y. Furusho and E. Yashima, Angew. Chem., Int. Ed., 2010, 49, 5463–5467 CrossRef CAS PubMed.
- (a) M. Yamazaki, T. Hagiwara, M. Sekiguchi, T. Sawaguchi and S. Yano, Synth. Commun., 2008, 38, 553–558 CrossRef CAS; (b) T. J. Burchell, D. J. Eisler and R. J. Puddephatt, Dalton Trans., 2005, 268–272 RSC; (c) M. Koizumi, C. Dietrich-Buchecker and J.-P. Sauvage, Eur. J. Org. Chem., 2004, 770–775 CrossRef CAS; (d) P. R. Ashton, A. M. Heiss, D. Pasini, F. M. Raymo, A. N. Shipway, J. F. Stoddart and N. Spencer, Eur. J. Org. Chem., 1999, 995–1004 CrossRef CAS.
- N. Kameta, Y. Nagawa, M. Karikomi and K. Hiratani, Chem. Commun., 2006, 3714–3716 RSC.
- (a) L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed; (b) R. J. Phipps, G. L. Hamilton and F. D. Toste, Nat. Chem., 2012, 4, 603–614 CrossRef CAS PubMed; (c) L. A. Joyce, M. S. Maynor, J. M. Dragna, G. M. da Cruz, V. M. Lynch, J. W. Canary and E. V. Anslyn, J. Am. Chem. Soc., 2011, 133, 13746–13752 CrossRef CAS PubMed; (d) J. Lacour and D. Moraleda, Chem. Commun., 2009, 7073–7089 RSC; (e) Z. Dai, X. Xu and J. W. Canary, Chirality, 2005, 17, S227–S233 CrossRef CAS PubMed.
- (a) T. Akiyama and K. Mori, Chem. Rev., 2015, 15, 9277–9306 CrossRef PubMed; (b) L. Liu, M. Leutzsch, Y. Zheng, M. W. Alachraf, W. Thiel and B. List, J. Am. Chem. Soc., 2015, 137, 13268–13271 CrossRef CAS PubMed; (c) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS PubMed.
- T. R. Wu, L. Shen and J. M. Chong, Org. Lett., 2004, 6, 2701–2704 CrossRef CAS PubMed.
- In order to prevent chemical shift changes by proton transfer from phosphoric acid to amine, we used the respective phosphate- and ammonium-salts in the titration experiments.
- Although H-7 and H-8 only show small Δδ-values upon guest binding, they do not overlap with other signals over the whole titration range (as opposed to H-6/9/12/13). Analysis of Δδ for H-7 and H-8 gave almost identical results.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc01980c |
| ‡ MS/MS-experiments. |
| This journal is © The Royal Society of Chemistry 2016 |