Open Access Article
Open Access ArticleConformational changes and chiroptical switching of enantiopure bis-helicenic terpyridine upon Zn2+ binding†
Helena
Isla
a,
Monika
Srebro-Hooper
*b,
Marion
Jean
c,
Nicolas
Vanthuyne
c,
Thierry
Roisnel
a,
Jamie L.
Lunkley
d,
Gilles
Muller
d,
J. A. Gareth
Williams
 e,
Jochen
Autschbach
f and
Jeanne
Crassous
*a
e,
Jochen
Autschbach
f and
Jeanne
Crassous
*a
aInstitut des Sciences Chimiques de Rennes, UMR 6226, Campus de Beaulieu, CNRS-Université de Rennes 1, 35042 Rennes Cedex, France. E-mail: jeanne.crassous@univ-rennes1.fr
bDepartment of Theoretical Chemistry, Faculty of Chemistry, Jagiellonian University, 30-060 Krakow, Poland. E-mail: srebro@chemia.uj.edu.pl
cAix Marseille Université, Centrale Marseille, CNRS, iSm2 UMR 7313, 13397, Marseille, France
dDepartment of Chemistry, San José State University, San José, CA 95192-0101, USA
eDepartment of Chemistry, University of Durham, Durham, DH1 3LE, UK
fDepartment of Chemistry, University at Buffalo, State University of New York, Buffalo, NY 14260, USA
First published on 25th March 2016
Abstract
The molecular conformation of a bis-helicenic terpyridine system is strongly modified upon binding to Zn(II) ion, a process that is accompanied by large changes in the optical and chiroptical properties. This system affords a new type of helicene-based chiroptical switching.
The contribution of chiroptical molecular switches1 to the field of functional devices is stimulated by many potential applications such as molecular information processing, data storage, or probes for the detection of chirality.2 Interconversion between two or more stable chiral forms can result from responses of chiral molecules to external stimuli such as heat, light, pressure, solvent, pH, change in redox potential or presence of chemicals. The response at the molecular level can be transduced to a read-out process through the effect on chiroptical activity; e.g., optical rotation, electronic circular dichroism (ECD), and circularly polarized luminescence (CPL). Efficient, practicable switching between two (or more) molecular states, is based on stability, non-destructive read-out, fast response times, reproducibility and fatigue resistance. An important requirement is a large response difference between the states to ensure unambiguous read-out.
In the last decade, helicenes have appeared as attractive candidates for molecular materials and chiroptical switches due to their helical π-conjugated structure which gives rise to strong chiroptical properties such as huge optical rotation values, intense ECD spectra and significant CPL activity.3 Examples of helicene-based chiroptical switches have been recently reviewed.4 However, the creation of structural diversity is still necessary to develop new kinds of functions in helicene-based chiral systems and to create innovative molecular devices. In this regard, several transition-metal complexes formed from helical π-conjugated ligands have recently been shown to display unprecedented chiroptical and electronic properties that are switchable by redox or acid/base stimuli.5 While in those examples the helical topology and integrity of the chemical backbone was maintained upon switching, constructing a scaffold in which movements can take place through geometrical and conformational changes is particularly appealing. Such conformational changes in chiral systems may lead to molecular motors with effective unidirectional motion.2,6
In this communication, we describe the synthesis of the first bis-helicenic terpyridine ligand (meso-, (M,M)- and (P,P)-1 in Scheme 1) acting as a chiroptical switch upon reversible coordination–decoordination of zinc(II). The profound conformational changes induced lead to a multi-output read-out system, showing responses in ECD, fluorescence and CPL-activity. The (chir)optical changes accompanying the interconversion between the ligand and zinc-complexed states have been analyzed via first-principles calculations.
 | ||
| Scheme 1 Synthesis of bis-helicenic terpyridine 1. (i) n-BuLi, THF, r.t., 5 h, 82%; (ii) hν, cat. I2, toluene/THF, r.t., 7 h, 45%. | ||
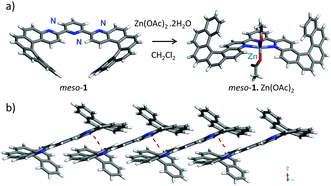
The synthesis of ligand 1 was accomplished as described in Scheme 1. First, a Wittig reaction between terpyridine bis-aldehyde 27a and (benzo[c]phenanthren-3-ylmethyl)triphenyl-phosphonium bromide 37b yielded bis-olefin 4 as a mixture of three isomers (E,E – major compound, Z,Z, and E,Z). Subsequent photo-irradiation (700 W Hg lamp) in a toluene/THF mixture at room temperature (r.t.) in the presence of catalytic iodine, furnished bis-helicene-terpyridine 1 as a statistical mixture of (M,P)-1 (meso-1) and racemic (P,P)- and (M,M)-1. The structure of meso-1 was confirmed by NMR spectroscopy and by X-ray diffraction studies. The 1H NMR spectrum is in agreement with its Cs symmetry and displays the typical signature of [6]helicene derivatives such as for example degenerate triplets at 6.8 and 7.3 ppm corresponding to H15 and H14, respectively (see ESI†). Thanks to its low solubility, meso-1 easily crystallized in the centrosymmetric Ccm21 space group, with the nitrogen atoms of adjacent pyridyl rings disposed trans to one another, as is usual in 2,2′:6′,2′′-terpyridines (Scheme 2a).8 The structure also confirms (i) the Cs symmetry of the achiral meso compound; (ii) the high degree of planarity of the terpy moiety (dihedral angles between the different pyridine rings <6.5°); and (iii) values for the helical angle (angle between the terminal rings) of the azahelicene moieties that are typical of such units (51.83°).3
Pure samples of (M,M)- and (P,P)-1 were successfully obtained from the crude stereoisomeric mixture by using HPLC over a chiral stationary phase and were then fully characterized (see ESI†). They display different 1H NMR spectra from the meso-1 compound in agreement with different orientation of helicenic moieties (for instance H15 and H14 resonate at 6.7 and 7.1, ppm, respectively). The X-ray structure of (P,P)-1 (P21221 space group; Scheme 2b) shows (i) C2 symmetry as opposed to the Cs symmetry of the meso isomer; (ii) again a high degree of planarity within the terpy moiety (dihedral angles between the pyridine rings <8.3°) and a trans disposition of N atoms in adjacent rings; and (iii) typical values for the helical angle of the azahelicene moieties (60.84°).3 Furthermore, compound (P,P)-1 displays intermolecular organization along the x axis thanks to the presence of π-stacking interactions (centroid–centroid distances 3.67 Å; Scheme 2b).
Compounds meso-1 and (P*,P*)-1‡ display photophysical properties with similar characteristics to one another (see ESI†). For example, the UV-visible spectra of these two compounds show a very strong absorption band centred at about 320 nm (ε ∼ 6 × 104 M−1 cm−1) and a set of bands extending into the visible region, of gradually diminishing intensity, of which the longest wavelength appears at 416 nm (ε = 5–8 × 103 M−1 cm−1). (P*,P*)-1‡ displays intense blue fluorescence in CH2Cl2 at r.t. (λmax = 421 nm, ϕ = 8.4%, τ = 5.7 ns). Its structured emission spectrum is characterised by a vibronic progression of ∼1350 cm−1, with the maximum intensity being in the 0–0 band, indicative of a rigid aromatic fluorophore. In a frozen glass at 77 K, the fluorescence is accompanied by long-lived green phosphorescence (λmax = 532 nm, τ = 1.4 s), with similar vibrational structure. Similar spectra were recorded for meso-1. The emission properties are in line with previously described nitrogen-containing [6]helicene derivatives.5d
The chiroptical properties of (M,M)- and (P,P)-1 were then studied. High specific and molar optical rotation values were obtained ((P,P)-1: [α]23D = +2150 [°/(dm g/cm−3)], [ϕ]23D = +15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 [° cm2 dmol−1] (±5%, CH2Cl2, 2.2 × 10−4 M)). The molar rotation is twice as high as for 4-aza[6]helicene ([α]23D = +2290, [ϕ]23D = +7735 (±5%, CH2Cl2, C 1.7 g/100 mL))9 in agreement with the presence of two azahelicene moieties. Similarly, very strong mirror-image ECD spectra were recorded for (M,M)- and (P,P)-1 enantiomers (Fig. 1), with a strong negative band around 264 nm (Δε = −280 M−1 cm−1) and strong positive one at 341 (+388 M−1 cm−1) for the (P,P)-1 enantiomer. Interestingly, a clear substructure is appearing and is assigned to a vibronic progression (∼1350 cm−1, C
790 [° cm2 dmol−1] (±5%, CH2Cl2, 2.2 × 10−4 M)). The molar rotation is twice as high as for 4-aza[6]helicene ([α]23D = +2290, [ϕ]23D = +7735 (±5%, CH2Cl2, C 1.7 g/100 mL))9 in agreement with the presence of two azahelicene moieties. Similarly, very strong mirror-image ECD spectra were recorded for (M,M)- and (P,P)-1 enantiomers (Fig. 1), with a strong negative band around 264 nm (Δε = −280 M−1 cm−1) and strong positive one at 341 (+388 M−1 cm−1) for the (P,P)-1 enantiomer. Interestingly, a clear substructure is appearing and is assigned to a vibronic progression (∼1350 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands). A weak positive ECD band (Δε = +21 M−1 cm−1) is also present at 420 nm.
C stretching bands). A weak positive ECD band (Δε = +21 M−1 cm−1) is also present at 420 nm.
 | ||
| Fig. 1 Experimental ECD spectra of enantiopure (M,M)- and (P,P)-1 and its Zn(II) complex (P,P)-1·Zn(OAc)2 (CH2Cl2, 2 × 10−5 M). Inset: Simulated spectra of (P,P)-1 (trans and cis) and (P,P)-1·Zn(OAc)2 (see ESI†). | ||
CPL activity is another appealing chiroptical property of organic chiral chromophores such as helicenic derivatives and has been examined recently by us and others.10 Indeed (M,M)- and (P,P)-1 enantiomers demonstrated mirror-imaged CPL spectra (Fig. S20, ESI†) with opposite glum values ((P,P)-1: +8.6 × 10−3 and (M,M)-1: −8.4 × 10−3) around the emission maximum (∼420 nm). These values are slightly stronger than for related molecules such as helicene-bipyridine derivatives (glum ∼ ±10−3)5d or other CPL-active organic molecules.11
The terpyridine system is a good scaffold for coordination to a variety of transition-metal centers yielding complexes with a rich variety of photophysical properties that may be appealing for device applications.8,12 Rarely have chiral substituents been integrated within the terpy scaffold and used as chiroptical switches or chiral sensors.13 In 2004, Lehn et al. described a two-state switching system based on a terpyridine-type receptor which was activated towards substrate binding upon complexation of a zinc(II) cation.12a This process was based on so-called “nanomechanical motions” induced by ion binding and involving shape switching between two forms presenting different physicochemical properties, namely the metal-bound and the metal-free states. Inspired by this seminal work, we explored how Zn(II) binding to 1 could induce chemical switching of the (chir)optical properties through the large conformational changes associated with the switch from W- to U-shape geometries of the terpy moiety (Fig. 2b).
 | ||
Fig. 2 (a) Reversible Zn(II) complexation–decomplexation process of (P,P)-1 using Zn(OAc)2 and TPEN as the chemical stimuli. (b) Schematic modification of the geometry. (c) Evolution of the UV-vis spectrum of (P*,P*)-1‡ (3 × 10−5 M, CH2Cl2, r.t.) upon addition of Zn(OAc)2·2H2O aliquots (0.2 equiv. until saturation at 1.0–1.2 equiv.). Inset: Absorption at 432 nm and fitting with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. (d) Evolution of the ECD spectrum of (P,P)-1. Inset: Reversible ECD341 1 binding model. (d) Evolution of the ECD spectrum of (P,P)-1. Inset: Reversible ECD341![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm switching process. (e) Evolution of the fluorescence of (P,P)-1 (λex = 350 nm, 2.2 × 10−5 M, CH2Cl2, r.t.). nm switching process. (e) Evolution of the fluorescence of (P,P)-1 (λex = 350 nm, 2.2 × 10−5 M, CH2Cl2, r.t.). | ||
In the first instance, meso-1·Zn(OAc)2 complex was prepared from meso-1 ligand upon treatment with 1 equiv. of Zn(OAc)2·2H2O in CH2Cl2 (see Scheme 2a). This complex was obtained quantitatively (Ka = 1.98 × 106 M−1) and was fully characterized (see ESI†). Single crystals could be grown and the X-ray structure (P121/n1 space group, Scheme 2a) shows the pentacoordinated Zn centre, with two OAc and the terpy ligand in a cis geometry (U shape). Note that upon coordination, the meso-1 ligand has kept its Cs symmetry but the helical angle of the aza[6]helicene parts has increased to 77.72°, certainly due to steric hindrance. Overall, upon coordination to the metal ion, the two aza[6]helicene moieties have fully rotated around the C–C bonds from one side of the central pyridyl ring to the other, thus inducing the anticipated W- to U-shape conformational change.
In a second stage, aliquots of Zn(OAc)2·2H2O were added to (M,M)- and (P,P)-1 and the complexation process (Fig. 2a and b) was followed by UV-vis and ECD spectroscopy (0.2 equivalents of Zn(OAc)2 were added until saturation at 1.0–1.2 equiv.). Large changes were observed in the UV-vis spectrum of (P*,P*)-1‡ upon Zn(II) binding, as depicted in Fig. 2c, with the presence of several isosbestic points, showing that the two species are in equilibrium. The solution turned from pale yellow to dark yellow colour. Curve fitting of the absorption variation at 432 nm revealed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model with a high association constant (Ka = 1.16 × 106 M−1). Substantial modifications were also observed for chiroptical properties of (P,P)-1 upon Zn(II) binding (see Fig. 1 and 2d). For example, the strong, positive ECD-active band at 341 nm decreases from +388 to +220 M−1 cm−1, a band around 310 nm increases and gains significant positive intensity (from −70 to +90 M−1 cm−1), and a new positive band of low intensity appears at 430 nm (Δε = +26 M−1 cm−1), while the molar rotation slightly increases ((P,P)-1·Zn(OAc)2: [α]23D = +2300, [ϕ]23D = +17
1 binding model with a high association constant (Ka = 1.16 × 106 M−1). Substantial modifications were also observed for chiroptical properties of (P,P)-1 upon Zn(II) binding (see Fig. 1 and 2d). For example, the strong, positive ECD-active band at 341 nm decreases from +388 to +220 M−1 cm−1, a band around 310 nm increases and gains significant positive intensity (from −70 to +90 M−1 cm−1), and a new positive band of low intensity appears at 430 nm (Δε = +26 M−1 cm−1), while the molar rotation slightly increases ((P,P)-1·Zn(OAc)2: [α]23D = +2300, [ϕ]23D = +17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (±5%, CH2Cl2, 2.18 × 10−4 M), vide supra). Similarly, the luminescence profile of 1 significantly changes upon adding Zn(OAc)2, with the appearance of a structureless intense fluorescence emission at 480 nm (ϕ = 19%, τ = 3.4 ns, see Fig. 2e) and CPL activity at this wavelength with glum values of +1.2 × 10−3 and −1.4 × 10−3 for (P,P)-1·Zn(OAc)2 and (M,M)-1·Zn(OAc)2, respectively (see ESI†). The emission colour turned from blue to green.
300 (±5%, CH2Cl2, 2.18 × 10−4 M), vide supra). Similarly, the luminescence profile of 1 significantly changes upon adding Zn(OAc)2, with the appearance of a structureless intense fluorescence emission at 480 nm (ϕ = 19%, τ = 3.4 ns, see Fig. 2e) and CPL activity at this wavelength with glum values of +1.2 × 10−3 and −1.4 × 10−3 for (P,P)-1·Zn(OAc)2 and (M,M)-1·Zn(OAc)2, respectively (see ESI†). The emission colour turned from blue to green.
Calculations at the LC-PBE0*/SV(P) level14 with a continuum solvent model for CH2Cl2 reproduce these modifications well and link them predominantly to the large conformational changes in the structure of the ligand 1 upon Zn(II) coordination. The simulated ECD spectrum of ligand 1 in the cis conformation of the terpy unit indeed clearly resembles the one calculated for 1·Zn(OAc)2 (Fig. 1). Additionally, the Zn(II) complexation has a strong effect on the LUMO and LUMO+1 of 1 (both π*), leading to a concentration of their density around the zinc ion in the terpyridine part of the ligand (see ESI† for the MO analysis).5d The HOMO and other high lying occupied π-orbitals remain helicene-centered and therefore the complexation strongly increases the character of charge-transfer from the helicene to the terpyridine part of the ligand in the low-energy part of the spectrum.5d Note that Zn orbitals were not found to contribute to the π-conjugated system of the helicene ligand, as expected for closed-shell d10 metal centers. The primary effect of the Zn moiety is therefore to activate the structural transformation of the aza[6]helicene fragments, rigidify the system, and induce charge-transfer excitations within the ligand. The calculations also correctly reproduce changes in the S1–S0 (HOMO–LUMO) emission upon Zn(II) complexation, which is in line with the noticeable energetic stabilization of the LUMO in the complex relative to the pristine ligand: from 419 nm, glum = +6.8 × 10−3 computed for (P,P)-1 to 452 nm, glum = +1.1 × 10−3 for (P,P)-1·Zn(OAc)2 (vide supra, see ESI†).
In order to verify the reversibility of the Zn(II) binding process, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) was utilized as a competitive ligand for zinc: upon treatment with TPEN, the system returned to its original state, i.e. to the native chiral (P,P)- and (M,M)-1 ligands. Up to 9–10 steps could be performed upon successive additions of 1 equiv. of Zn(OAc)2 and TPEN, following the ECD tuning at a selected wavelength where the changes are very high (for example ΔΔε = 175 M−1 cm−1 at 341 nm, see inset of Fig. 2d). Overall, this system is behaving as a chiroptical switch offering multi-output read-out (UV-vis, ECD, luminescence and CPL). Furthermore, the switching process triggers conformational changes and molecular motion around the Zn centre, from a clear trans (W-shape) conformation in the free ligand to a cis (U-shape) one in the Zn-complex. To our knowledge, this constitutes a new type of helicene-based chiroptical switching.4 In conclusion, the preparation of unprecedented bis-helicenic terpy ligand has opened new perspectives in multifunctional chiral complexes and chiroptical switches; coordination to other metallic ions is under current investigation.
We thank the Ministère de l'Education Nationale, de la Recherche et de la Technologie, the Centre National de la Recherche Scientifique (CNRS), the ANR (12-BS07-0004-METALHEL-01), the Région Bretagne (SAD CHIROPEM) and the LIA Rennes-Durham for financial support. M. S.-H. is grateful for financial support from the Ministry of Science and Higher Education in Poland (‘Outstanding Young Scientist’ scholarship). J. A. acknowledges the National Science Foundation (CHE 1265833) for financial support and the Center for Computational Research (CCR) at the University at Buffalo for computational resources. G. M. thanks the NIH, Minority Biomedical Research Support (grant 1 SC3 GM089589-07) and the Henry Dreyfus Teacher-Scholar Award for financial support.
Notes and references
- (a) J. W. Canary, S. Mortezaei and J. Liang, Coord. Chem. Rev., 2010, 254, 2249 CrossRef CAS; (b) J. W. Canary and Z. Dai, Dynamic stereochemistry and chiroptical spectroscopy of metallo-organic compounds, in Comprehensive Chiroptical Spectroscopy, ed. N. Berova, R. W. Woody, P. Polavarapu and K. Nakanishi, John Wiley & Sons, Hoboken, NJ, USA, 2012, vol. 2 Search PubMed; (c) H. Miyake and H. Tsukube, Chem. Soc. Rev., 2012, 41, 6977 RSC; (d) Z. Dai, J. Lee and W. Zhang, Molecules, 2012, 17, 1247 CrossRef CAS PubMed; (e) J. Crassous, Chem. Commun., 2012, 48, 9684 RSC.
- (a) B. L. Feringa and W. R. Browne, Molecular Switches, Wiley-VCH, 2nd edn, 2011 Search PubMed; (b) V. Balzani, A. Credi and M. Venturi, Molecular Devices and Machines, Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, 2008 Search PubMed.
- Selected recent reviews: (a) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS PubMed; (b) M. Gingras, Chem. Soc. Rev., 2013, 42, 1051 RSC; (c) N. Saleh, C. Shen and J. Crassous, Chem. Sci., 2014, 5, 3680 RSC; (d) J. Bosson, J. Gouin and J. Lacour, Chem. Soc. Rev., 2014, 43, 2824 RSC.
- For a review see: H. Isla and J. Crassous, C. R. Chim., 2016, 19, 39 CrossRef CAS and references therein.
- (a) E. Anger, M. Srebro, N. Vanthuyne, L. Toupet, S. Rigaut, C. Roussel, J. Autschbach, J. Crassous and R. Réau, J. Am. Chem. Soc., 2012, 134, 15628 CrossRef CAS PubMed; (b) E. Anger, M. Srebro, N. Vanthuyne, C. Roussel, L. Toupet, J. Autschbach, R. Réau and J. Crassous, Chem. Commun., 2014, 50, 2854 RSC; (c) M. Srebro, E. Anger, B. Moore, II, N. Vanthuyne, C. Roussel, R. Réau, J. Autschbach and J. Crassous, Chem. – Eur. J., 2015, 21, 17100 CrossRef CAS PubMed; (d) N. Saleh, B. Moore, II, M. Srebro, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, K. K. Deol, G. Muller, J. Autschbach and J. Crassous, Chem. – Eur. J., 2015, 21, 1673 CrossRef CAS PubMed.
- Selected: (a) T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150 CrossRef CAS PubMed; (b) N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS PubMed; (c) J. V. Hernandez, E. R. Kay and D. A. Leigh, Science, 2004, 306, 1532 CrossRef CAS PubMed; (d) R. A. van Delden, M. K. J. ter Wiel, M. M. Pollard, J. Vicario, N. Koumura and B. L. Feringa, Nature, 2005, 437, 1337 CrossRef CAS PubMed; (e) K. Miwa, Y. Furusho and E. Yashima, Nat. Chem., 2010, 2, 444 CrossRef CAS PubMed; (f) H. L. Tierney, C. J. Murphy, A. D. Jewell, A. E. Baber, E. V. Iski, H. Y. Khodaverdian, A. F. McGuire, N. Klebanov and E. C. H. Sykes, Nat. Nanotechnol., 2011, 6, 625 CrossRef CAS PubMed; (g) U. G. E. Perera, F. Ample, H. Kersell, Y. Zhang, G. Vives, J. Echeverria, M. Grisolia, G. Rapenne, C. Joachim and S.-W. Hla, Nat. Nanotechnol., 2013, 8, 46 CrossRef CAS PubMed; (h) G. Haberhauer, Angew. Chem., Int. Ed., 2008, 47, 3635 CrossRef CAS PubMed; (i) Y. Suzuki, T. Nakamura, H. Iida, N. Ousaka and E. Yashima, J. Am. Chem. Soc., 2016 DOI:10.1021/jacs.6b00787.
- (a) Y. D. M. Champouret, R. K. Chaggar, I. Dadhiwala, J. Fawcett and G. A. Solan, Tetrahedron, 2006, 62, 79 CrossRef CAS; (b) D. A. Lightner, D. T. Hefelfinger, T. W. Powers, G. W. Frank and K. N. Trueblood, J. Am. Chem. Soc., 1972, 94, 3492 CrossRef CAS.
- A. Wild, A. Winter, F. Schlüttera and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459 RSC.
- D. Mendola, N. Saleh, N. Vanthuyne, C. Roussel, L. Toupet, F. Castiglione, T. Caronna, A. Mele and J. Crassous, Angew. Chem., Int. Ed., 2014, 53, 5786 CrossRef CAS PubMed.
- Selected examples of CPL active helicenes: (a) J. E. Field, G. Muller, J. P. Riehl and D. Venkataraman, J. Am. Chem. Soc., 2003, 125, 11808 CrossRef CAS PubMed; (b) Y. Sawada, S. Furumi, A. Takai, M. Takeuchi, K. Noguchi and K. Tanaka, J. Am. Chem. Soc., 2012, 134, 4080 CrossRef CAS PubMed; (c) K. E. S. Phillips, T. J. Katz, S. Jockusch, A. J. Lovinger and N. J. Turro, J. Am. Chem. Soc., 2001, 123, 11899 CrossRef CAS PubMed; (d) T. Kaseyama, S. Furumi, X. Zhang, K. Tanaka and M. Takeuchi, Angew. Chem., Int. Ed., 2011, 50, 3684 CrossRef CAS PubMed; (e) C. Shen, E. Anger, M. Srebro, N. Vanthuyne, K. K. Deol, T. D. Jefferson Jr., G. Muller, J. A. G. Williams, L. Toupet, C. Roussel, J. Autschbach, R. Réau and J. Crassous, Chem. Sci., 2014, 5, 1915 RSC; (f) K. Nakamura, S. Furumi, M. Takeuchi, T. Shibuya and K. Tanaka, J. Am. Chem. Soc., 2014, 136, 5555 CrossRef CAS PubMed; (g) N. Saleh, M. Srebro, T. Reynaldo, N. Vanthuyne, L. Toupet, V. Y. Chang, G. Muller, J. A. G. Williams, C. Roussel, J. Autschbach and J. Crassous, Chem. Commun., 2015, 51, 3754 RSC; (h) Y. Yamamoto, H. Sakai, J. Yuasa, Y. Araki, T. Wada, T. Sakanoue, T. Takenobu, T. Kawai and T. Hasobe, Chem. – Eur. J., 2016, 22, 4263 CrossRef CAS PubMed.
- See reviews: (a) H. Maeda and Y. Bando, Pure Appl. Chem., 2013, 85, 1967 CrossRef CAS; (b) E. M. Sanchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488 CrossRef CAS PubMed; (c) J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed.
- (a) A. Petitjean, R. G. Khoury, N. Kyritsakas and J.-M. Lehn, J. Am. Chem. Soc., 2004, 126, 6637 CrossRef CAS PubMed; (b) B. Doistau, A. Tron, S. A. Denisov, G. Jonusauskas, N. D. McClenaghan, G. Gontard, V. Marvaud, B. Hasenknopf and G. Vives, Chem. – Eur. J., 2014, 20, 15799 CrossRef CAS PubMed; (c) B. Doistau, J.-L. Cantin, L.-M. Chamoreau, V. Marvaud, B. Hasenknopf and G. Vives, Chem. Commun., 2015, 51, 12916 RSC; (d) A. Fermi, G. Bergamini, M. Roy, M. Gingras and P. Ceroni, J. Am. Chem. Soc., 2014, 136, 6395 CrossRef CAS PubMed.
- (a) M. Ziegler, V. Monney, H. Stoeckli-Evans, A. Von Zelewsky, I. Sasaki, G. Dupic, J.-C. Daran and G. G. A. Balavoine, J. Chem. Soc., Dalton Trans., 1999, 667 RSC; (b) S. Bernhard, K. Takada, D. J. Díaz, H. D. Abruña and H. Mürner, J. Am. Chem. Soc., 2001, 123, 10265 CrossRef CAS PubMed; (c) S. Bernhard, J. I. Goldsmith, K. takada and H. D. Abruña, Inorg. Chem., 2003, 42, 4389 CrossRef CAS PubMed; (d) A. Khatyr and R. Ziessel, Org. Lett., 2001, 3, 1857 CrossRef CAS PubMed; (e) S. Bezer, S. Rapireddy, Y. A. Skorik, D. H. Ly and C. Achim, Inorg. Chem., 2011, 50, 11929 CrossRef CAS PubMed.
- (a) J. Autschbach, Chirality, 2009, 21, E116 CrossRef CAS PubMed; (b) J. Autschbach, L. Nitsch-Velasquez and M. Rudolph, Top. Curr. Chem., 2011, 298, 1 CrossRef CAS PubMed; (c) J. Autschbach and M. Srebro, Acc. Chem. Res., 2014, 47, 2592 CrossRef CAS PubMed; (d) B. Pritchard and J. Autschbach, ChemPhysChem, 2010, 11, 2409 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic, spectroscopic and computational details. CCDC 1448892–1448894. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc01748g |
| ‡ (P*,P*)-1 means the racemic mixture of (P,P)- and (M,M)-1. |
| This journal is © The Royal Society of Chemistry 2016 |