Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intermolecular dearomative C2-arylation of N-Ac indoles activated by FeCl3†
Raj Kumar
Nandi
,
Friederike
Ratsch
,
Rodolphe
Beaud
,
Régis
Guillot
,
Cyrille
Kouklovsky
and
Guillaume
Vincent
 *
*
Univ Paris Sud, CNRS, Université Paris-Saclay, Institut de Chimie Moléculaire et des Matériaux d'Orsay (ICMMO), Equipe Méthodologie, Synthèse et Molécules Thérapeutiques (MS&MT), Orsay, 91405, France. E-mail: guillaume.vincent@u-psud.fr
First published on 11th March 2016
Abstract
We report the FeCl3-mediated direct addition of electron-rich arenes to the C2-position of electrophilic N-Ac indoles under mild conditions (room temperature, air). No functional group is required on the arene nucleophile: one of its C–H bonds is added to the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond of the indole nucleus in a Friedel–Crafts-type reaction. This dearomatisation process delivered a broad range of C2-arylated indolines.
C3 double bond of the indole nucleus in a Friedel–Crafts-type reaction. This dearomatisation process delivered a broad range of C2-arylated indolines.
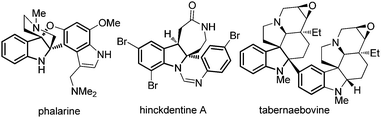
The functionalization of indole derivatives via dearomatisation reactions is a field of intense synthetic efforts due to the biological relevance of the heterocyclic scaffolds obtained.1 In this context, we have recently described several methods for the dearomative C3-arylation of indoles2via FeCl3-activation of 3-substituted N-Ac indoles3 or oxidation of indoles with NIS4 or from N-hydroxyindoles5 using the electrophilicity of indoles.6 Our next goal was to achieve the related C2-arylation of indoles due to the presence of the C2-arylindoline motif in several natural products such as phalarine, hinckdentine A or tabernaebovine (Fig. 1).7
The arylative dearomatisation of indoles also allows transformation of a flat heterocycle into a 3-D structure which is more poised to explore chemical space in the context of drug discovery.8
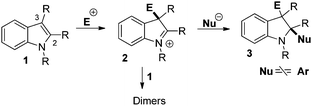
Indoles 1 are widely known to be highly nucleophilic at the C3-position leading, in the presence of electrophiles or acids, to indolium ions 2 which can be trapped by nucleophiles at the C2-position and delivered functionalized indolines 3, usually in the intramolecular mode (Scheme 1).9 Due to the propensity of indolium ions 2 to form dimeric compounds via attack of indole 1 at C2,10 intermolecular addition of aryl nucleophiles to indoliums such as 2 is very rare.11 Only a few nucleophiles such as allylboranes or hydrides could be added to 2 in the intermolecular mode.12
Usually, the C2-arylative dearomatisation of indoles relies on intramolecular reactions with the aryl substituent attached to the nitrogen of the indole nucleus. In this case, the aryl group is introduced by transformation of an aryl–halogen bond via palladium-catalysed13 or radical reactions.14
In the intermolecular mode, the C2-arylation of indoles could take place by the 1,4 addition of Grignard reagents to indoles containing a strong electron withdrawing group at C3.15 An elegant palladium-catalyzed 3-oxy-2-arylation of 3-unsubstituted indoles with phenylboronic acids and TEMPO was described.16 These methods rely on the use of a functional group on the aromatic nucleophile. Alternatively, during the total synthesis of dideepoxytabernaebovine, a rare and straightforward addition of electron rich arenes to an indolium intermediate such as 2 was deployed.11a A formal [4+2] cycloaddition with a N-phenyl iminium intermediate was also described.17 We achieved the 3-oxy-2-arylation of indoles during the DDQ-mediated oxidative coupling between phenols and 3-substituted N-Ac-indoles activated by FeCl3.18
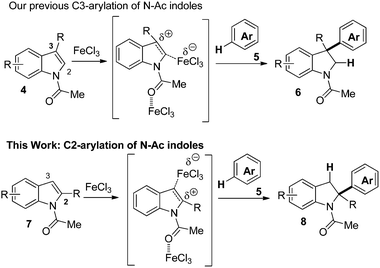
Despite these achievements, we felt that a general method for the C2-arylation of indoles by the functionalization of the C–H bond of the aromatic nucleophile was lacking. To achieve the C2-regioselective addition of a C–H bond across the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond of indoles, our experience in the activation of N-Ac-indoles by FeCl3 was crucial to induce a Friedel–Crafts process.3 We discovered that the FeCl3-mediated hydroarylation of 3-unsubstituted N-Ac-indoles proceeds at the C2-position. We can postulate that the complexation of FeCl3 by the oxygen of the acetyl leads to the sequestration of the nitrogen lone pair by conjugation, the C2
C3 double bond of indoles, our experience in the activation of N-Ac-indoles by FeCl3 was crucial to induce a Friedel–Crafts process.3 We discovered that the FeCl3-mediated hydroarylation of 3-unsubstituted N-Ac-indoles proceeds at the C2-position. We can postulate that the complexation of FeCl3 by the oxygen of the acetyl leads to the sequestration of the nitrogen lone pair by conjugation, the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 bond could then be activated by an acid species and formally lead to a positive charge at C2 or C3.3b If a substituent is present at C3, the positive charge could be more stabilized at C3 by the formation of a tertiary benzylic carbocation.3a,b If the C3 position lacks substitution, the resulting secondary benzylic carbocation at C3 would be less stabilized than a positive charge at C2, due to the effect of the lone pair of the nitrogen. The latter would lead to the C2-regioselective hydroarylation of indoles which is the subject of this report (Scheme 2).
C3 bond could then be activated by an acid species and formally lead to a positive charge at C2 or C3.3b If a substituent is present at C3, the positive charge could be more stabilized at C3 by the formation of a tertiary benzylic carbocation.3a,b If the C3 position lacks substitution, the resulting secondary benzylic carbocation at C3 would be less stabilized than a positive charge at C2, due to the effect of the lone pair of the nitrogen. The latter would lead to the C2-regioselective hydroarylation of indoles which is the subject of this report (Scheme 2).
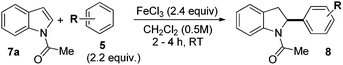
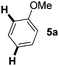
We started with N-Ac indole 7a as the electrophilic indole and we investigated a broad range of aromatic nucleophiles 5 at room temperature in dichloromethane in the presence of 2.4 equivalents of FeCl3 (Table 1). Anisole 5a reacts selectively at the C2-position of 7a in an 86% yield with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
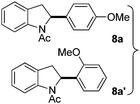
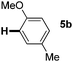
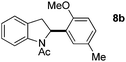
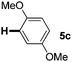
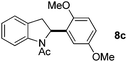
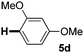
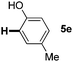
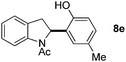
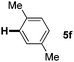
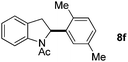
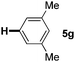
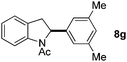
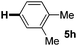
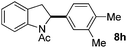
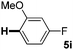
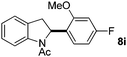
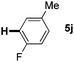
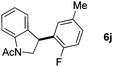
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of addition products at the para and ortho positions of 5a (entry 1). Arenes containing strong electron donating groups such as 4-methyl anisole 5b, 1,4-dimethoxybenzene 5c, 1,3-dimethoxybenzene 5d, and 4-methylphenol 5e delivered regioselectively and efficiently the C2-arylated N-Ac indoles 8b–e in 86–46% yields (entries 2–5).19 Xylenes 5f–h were also good partners for the regioselective C2-hydroarylation leading to 8f–h in 60–47% yields (entries 6–8). Fluorine-containing arene 5i also delivered 8i in 57% yield (entry 9). Finally, less electron rich arenes 5j,k surprisingly delivered moderate yields of 3-arylated indolines 6j,k (entries 10 and 11). It seems that the regioselectivity of the hydroarylation is also dependent on the arene nucleophile. The rationale for this observation is, presently, unclear to us.
1 mixture of addition products at the para and ortho positions of 5a (entry 1). Arenes containing strong electron donating groups such as 4-methyl anisole 5b, 1,4-dimethoxybenzene 5c, 1,3-dimethoxybenzene 5d, and 4-methylphenol 5e delivered regioselectively and efficiently the C2-arylated N-Ac indoles 8b–e in 86–46% yields (entries 2–5).19 Xylenes 5f–h were also good partners for the regioselective C2-hydroarylation leading to 8f–h in 60–47% yields (entries 6–8). Fluorine-containing arene 5i also delivered 8i in 57% yield (entry 9). Finally, less electron rich arenes 5j,k surprisingly delivered moderate yields of 3-arylated indolines 6j,k (entries 10 and 11). It seems that the regioselectivity of the hydroarylation is also dependent on the arene nucleophile. The rationale for this observation is, presently, unclear to us.
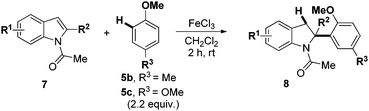
We then turned our attention to the scope of N-Ac indoles 7 with 4-methylanisole 5b and 1,4-dimethoxybenzene 5c as nucleophiles (Table 2).
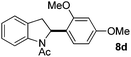
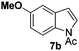
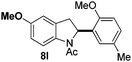
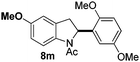
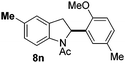
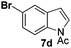
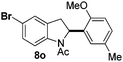
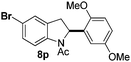
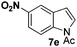
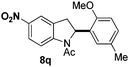
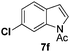
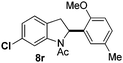
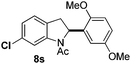
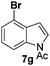
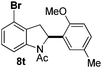
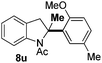
At the 5-position, indoles containing electron donating groups such as methoxy and methyl groups reacted efficiently with 5b delivering 8l,n in 67% and 74% yields (entries 1 and 3). The reaction between 5-methoxy-N-Ac indole 7b and 5c afforded moderate yield of 8m (entry 2). The 5-bromo-N-Ac indole reacted well with 5b,c leading to 8o,p in 83% and 54% yields (entries 4 and 5). Remarkably, a strong electron withdrawing group such as nitro at the 5-position afforded 40% yield of 8q (entry 6). At the 6-postion the chloro derivative 7f delivered 8r,s in 55% and 62% yields with 5b,c (entries 7 and 8). Substitution at the 4-position was also tolerated and 4-bromo-arylated indoline 8t was obtained in 85% yield (entry 9). Finally, indoline 8u, containing an arylated quaternary carbon, was obtained in 30% yield from 2-methyl-N-Ac indole 7h (entry 10). In this case, the hydroarylation reaction is in competition with the migration of the acetyl from the N-position to the C3-position.
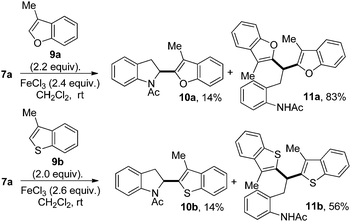
In order to obtain more diversified drug-like compounds, we studied the C2-addition of heterocycles such as benzofuran 9a or benzothiophene 9b to N-Ac indole 7a (Scheme 3).
Surprisingly, along with the expected C2-hydroarylated compounds 10a,b we also observed as the major products, compounds 11a,b.19
Compounds 11a,b most probably formed by the unexpected opening of the indole ring of 10a,b with the cleavage of the C2–NAc bond assisted by the lone-pair of the oxygen or sulfur atom. The resulting benzylic cationic intermediate could then be attacked by the addition of a second heteroarene. Such transformations have been observed during the trimerisation of indoles under acidic conditions.20
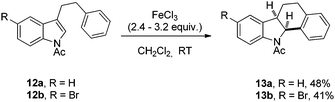
On a final note, we achieved the intramolecular 6-endo-trig arylation of 12a,b which contain an aryl group on the C3-side chain of the indole leading to tetracyclic compounds 13a,b arylated at the C2-position (Scheme 4).19 This is in contrast to the usual hydroarylation of 3-substituted N-Ac indoles which proceeds at the C3-position.3
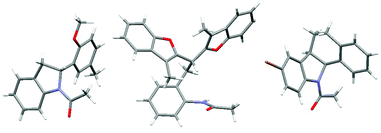
The structures of compounds 8b, 11a and 13b were confirmed by X-ray crystallography (Fig. 2).19
We have achieved the dearomative C2-arylation of N-Ac indoles by functionalization of the C–H bond of electron rich arenes at room temperature, in air with a cheap and non-toxic promoter. A broad scope of N-Ac indoles and arene nucleophiles is tolerated. Presumably, the key of this reaction is the generation of an electrophilic indole by activation of the N-Ac indole with FeCl3 which triggers a Friedel–Crafts reaction.
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013/ under REA grant agreement no. 623422 (IIF-2013 postdoctoral fellowship to R. K. N.). We also gratefully acknowledge the ANR (JCJC program 2012, ANR-12-JS07-0002; “CouPhIn”), the Université Paris Sud and the CNRS for financial support. The X-ray diffractometer was purchased with funds from Région Ile de France (SESAME program 2012, No. 12018501), IUF, LabEx CHARM3AT, Univ. Paris Sud and CNRS.
Notes and references
- S. P. Roche, J.-J. Youte Tendoung and B. Tréguier, Tetrahedron, 2015, 71, 3549–3591 CrossRef CAS.
- For a review: N. Denizot, T. Tomakinian, R. Beaud, C. Kouklovsky and G. Vincent, Tetrahedron Lett., 2015, 56, 4413–4429 CrossRef CAS.
- (a) R. Beaud, R. Guillot, C. Kouklovsky and G. Vincent, Angew. Chem., Int. Ed., 2012, 51, 12546–12550 CrossRef CAS PubMed; (b) R. Beaud, R. Guillot, C. Kouklovsky and G. Vincent, Chem. – Eur. J., 2014, 20, 7492–7500 CrossRef CAS PubMed; (c) R. Beaud, T. Tomakinian, N. Denizot, A. Pouilhès, C. Kouklovsky and G. Vincent, Synlett, 2014, 432–440 Search PubMed; (d) R. K. Nandi, R. Guillot, C. Kouklovsky and G. Vincent, submitted; for inspiring studies:; (e) K. Nishida, E. Yanase and S.-I. Nakatsuka, ITE Lett. Batteries, New Technol. Med., 2006, 7, 59–62 CAS.
- (a) N. Denizot, A. Pouilhès, M. Cucca, R. Beaud, R. Guillot, C. Kouklovsky and G. Vincent, Org. Lett., 2014, 16, 5752–5755 CrossRef CAS PubMed; (b) N. Denizot, R. Guillot, C. Kouklovsky and G. Vincent, Chem. – Eur. J., 2015, 21, 18953–18956 CrossRef CAS PubMed.
- T. Tomakinian, C. Kouklovsky and G. Vincent, Synlett, 2015, 1269–1275 CAS.
- M. Bandini, Org. Biomol. Chem., 2013, 11, 5206–5212 CAS.
- (a) P. A. Cockrum, S. M. Colegate, J. A. Edgar, K. Flower, D. Gardner and R. I. Willing, Phytochemistry, 1999, 51, 153–157 CrossRef; (b) A. J. Blackman, T. W. Hambley, K. Picker, W. C. Taylor and N. Thirasasana, Tetrahedron Lett., 1987, 28, 5561–5562 CrossRef CAS; (c) T. P. Lien, C. Kamperdick, T. Van Sung, G. Adam and H. Ripperger, Phytochemistry, 1998, 49, 1797–1799 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- (a) C. C. J. Loh and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 46–48 CrossRef CAS PubMed ; for selected examples: ; (b) S. A. Lakatosh, Y. N. Luzikov and M. N. Preobrazhenskaya, Org. Biomol. Chem., 2003, 1, 826–833 RSC; (c) S. A. Lakatosh, Y. N. Luzikov and M. N. Preobrazhenskaya, Tetrahedron, 2005, 61, 8241–8248 CrossRef CAS; (d) B. Han, Y.-C. Xiao, Y. Yao and Y.-C. Chen, Angew. Chem., Int. Ed., 2010, 49, 10189–10191 CrossRef CAS PubMed; (e) J.-J. Wang, A.-X. Zhou, G.-W. Wang and S.-D. Yang, Adv. Synth. Catal., 2014, 356, 3356–3362 CrossRef CAS.
- (a) H. F. Hodson and G. F. Smith, J. Chem. Soc., 1957, 3544–3545 RSC; (b) G. F. Smith and A. E. Walters, J. Chem. Soc., 1961, 940–943 RSC.
- (a) J. W. Medley and M. Movassaghi, Angew. Chem., Int. Ed., 2012, 51, 4572–4576 CrossRef CAS PubMed; (b) C. Charlet-Fagnère, J. Laronze, J.-Y. Laronze, L. Toupet, R. Vistelle, D. Lamiable, C. Mouchard, P. Renard and G. Adam, Bull. Soc. Chim. Fr., 1996, 1, 39–50 Search PubMed.
- (a) Y. N. Bubnov, I. V. Zhun', E. V. Klimkina, A. V. Ignatenko and Z. A. Starikova, Eur. J. Org. Chem., 2000, 3323–3327 CrossRef CAS; (b) F. Nowrouzi and R. A. Batey, Angew. Chem., Int. Ed., 2013, 52, 892–895 CrossRef CAS PubMed; (c) Y.-C. Xiao, C. Wang, Y. Yao, J. Sun and Y.-C. Chen, Angew. Chem., Int. Ed., 2011, 50, 10661–10664 CrossRef CAS PubMed.
- (a) L. Zhao, Z. Li, L. Chang, J. Xu, H. Yao and X. Wu, Org. Lett., 2012, 14, 2066–2069 CrossRef CAS PubMed; (b) C. Shen, R.-R. Liu, R.-J. Fan, Y.-L. Li, T.-F. Xu, J.-R. Gao and Y.-X. Jia, J. Am. Chem. Soc., 2015, 137, 4936–4939 CrossRef CAS PubMed; (c) D. A. Petrone, A. Yen, N. Zeidan and M. Lautens, Org. Lett., 2015, 17, 4838–4841 CrossRef CAS PubMed; (d) D. A. Petrone, M. Kondo, N. Zeidan and M. Lautens, Chem. – Eur. J., 2016 DOI:10.1002/chem.201600118.
- (a) S. Yasuda, T. Hirasawa, S. Yoshida and M. Hanaoka, Chem. Pharm. Bull., 1989, 37, 1682–1683 CAS; (b) W. Zhang and G. Pugh, Tetrahedron, 2003, 59, 3009–3018 CrossRef CAS; (c) A. S. Kyei, K. Tchabanenko, J. E. Baldwin and R. M. Adlington, Tetrahedron Lett., 2004, 45, 8931–8934 CrossRef CAS; (d) S. R. Flanagan, D. C. Harrowven and M. Bradley, Tetrahedron Lett., 2003, 44, 1795–1798 CrossRef CAS.
- L. Wang, Y. Shao and Y. Liu, Org. Lett., 2012, 14, 3978–3981 CrossRef CAS PubMed.
- (a) S. Kirchberg, R. Fröhlich and A. Studer, Angew. Chem., Int. Ed., 2009, 48, 4235–4238 CrossRef CAS PubMed; (b) V. Ramella, Z. He, C. G. Daniliuc and A. Studer, Org. Lett., 2015, 17, 664–667 CrossRef CAS PubMed.
- Z. Song, Y.-M. Zhao and H. Zhai, Org. Lett., 2011, 13, 6331–6333 CrossRef CAS PubMed.
- T. Tomakinian, R. Guillot, C. Kouklovsky and G. Vincent, Angew. Chem., Int. Ed., 2014, 53, 11881–11885 CrossRef CAS PubMed.
- CCDC 1450298 (8b), 1450300 (11b) and 1450299 (13b).
- W. Noland and W. Kuryla, J. Org. Chem., 1960, 25, 486–487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation and NMR spectra of new compounds and X-ray data. CCDC 1450298–1450300. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc01654e |
| This journal is © The Royal Society of Chemistry 2016 |