Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic conversion of methanol/ethanol to isobutanol – a highly selective route to an advanced biofuel†
Richard L.
Wingad
,
Emilie J. E.
Bergström
,
Matthew
Everett
,
Katy J.
Pellow
and
Duncan F.
Wass
*
School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: duncan.wass@brisol.ac.uk; Web: http://www.wassresearchgroup.com
First published on 11th March 2016
Abstract
Catalysts based on ruthenium diphosphine complexes convert methanol/ethanol mixtures to the advanced biofuel isobutanol, with extremely high selectivity (>99%) at good (>75%) conversion via a Guerbet-type mechanism.
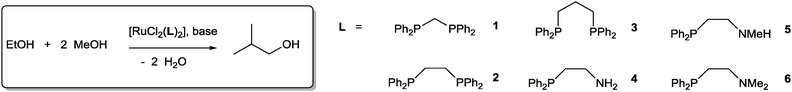
Obtaining liquid fuels for transportation from renewable biomass sources is an important element of future energy provision.1 (Bio)ethanol has long been used as a sustainable replacement for conventional gasoline, often in the form of a blend of the two. However, ethanol has a number of significant drawbacks compared to gasoline: it has a lower energy density (70% that of gasoline), it can be corrosive to current engine technology and fuel infrastructure, and it readily absorbs water leading to separation and dilution problems in storage tanks. Higher alcohols such as butanol are often termed advanced biofuels since they have fuel properties that more closely resemble those of gasoline and can alleviate many of these problems associated with ethanol.2 However, the bulk synthesis of butanol from biosustainable feedstocks remains challenged by low conversion and variable selectivity.3 We recently reported a new family of homogeneous ruthenium-based catalysts which demonstrate excellent performance in the upgrading of ethanol to n-butanol, with over 94% selectivity at good conversion.4 Key to this high selectivity was the use of small bite angle 1,1-bis(diphenylphosphino)methane (dppm) ligands, with larger bite angle diphosphines being less effective; mixed donor P–N ligands also give excellent results.5 Other homogeneous catalysts with comparable performance have also more recently been reported.6
Although n-butanol is a superior fuel to ethanol, the branched isomer isobutanol has even more desirable characteristics,7 and we have been exploring catalytic routes to this fuel molecule.
Our approach for n-butanol synthesis was to use ‘Guerbet’ type catalysts that allow facile C–C bond formation using normally unreactive alcohols;8 more broadly, reactions of this type are often termed ‘Borrowed Hydrogen’ chemistry.9 It is not obvious how this chemistry could be adapted for the direct conversion of ethanol alone to isobutanol; however, the co-condensation of methanol (which could also be obtained via biosustainable sources) and ethanol is an attractive potential route.10 Using these substrates, methanol and ethanol are dehydrogenated to formaldehyde and acetaldehyde, which undergo aldol coupling to yield, after re-hydrogenation, n-propanol. A further dehydrogenation, aldol coupling, re-hydrogenation cycle with methanol yields isobutanol (Scheme 1). Clearly, achieving high selectivity to isobutanol rather than the various other possible alcohol coupling products (for example, ethanol–ethanol to n-butanol) is crucial to a viable process.
Initially, we screened a variety of the ruthenium systems11 that have shown promise in ethanol homocoupling based on bis chelate diphosphine and mixed donor P–N ligand complexes.
Reaction conditions are similar to those used before, with NaOMe base, 180 °C and a 2 hour run time; in line with previous studies for isobutanol synthesis a higher concentration of base was typically used.10c An excess of methanol (molar methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol 14.4
ethanol 14.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)‡ was chosen to minimise possible ethanol homocoupling. Results are given in Table 1.
1)‡ was chosen to minimise possible ethanol homocoupling. Results are given in Table 1.
| Runa | Catalyst | Base | EtOH conversionb (%) | Selectivityc (%) | ||
|---|---|---|---|---|---|---|
| Isobutanol | n-Propanol | n-Butanol | ||||
| a Conditions: 1 mL (17.13 mmol) ethanol, 10 mL (247.13 mmol) methanol, 0.1 mol% [Ru], 200 mol% base (mol% based on ethanol substrate), 180 °C, 2 h. b Conversion of ethanol based on total amounts of liquid products obtained as determined by GC. c Total selectivity to products in the liquid fraction determined by GC. d 20 h. e 150 mol% base. f 100 mol% base. g 50 mol% base. h 120 °C, 20 h. i 150 °C, 20 h. j 180 °C, 20 h. k 0.62 mL water added (200 mol% based on ethanol substrate). | ||||||
| 1 | 1 | NaOMe | 66.4 | 98.1 | 1.8 | 0.1 |
| 2 | 2 | NaOMe | 3.3 | 95.4 | 4.6 | — |
| 3 | 3 | NaOMe | 4.7 | 59.2 | 40.8 | — |
| 4 | 4 | NaOMe | 41.6 | 92.3 | 7.3 | 0.4 |
| 5d | 4 | NaOMe | 56.3 | 89.7 | 9.9 | 0.4 |
| 6d | 5 | NaOMe | 48.6 | 95.5 | 4.2 | 0.3 |
| 7d | 6 | NaOMe | 31.1 | 93.2 | 6.5 | 0.3 |
| 8 | 1 | NaOtBu | 55.2 | 98.0 | 1.9 | 0.1 |
| 9 | 1 | NaOH | 73.8 | 95.6 | 4.4 | 0.1 |
| 10 | 1 | KOH | 60.8 | 94.9 | 4.9 | 0.2 |
| 11e | 1 | NaOMe | 69.6 | 97.5 | 2.5 | — |
| 12f | 1 | NaOMe | 49.9 | 93.6 | 6.2 | 0.2 |
| 13g | 1 | NaOMe | 12.0 | 90.2 | 8.9 | 0.8 |
| 14h | 1 | NaOMe | 11.2 | 81.2 | 18.6 | 0.3 |
| 15i | 1 | NaOMe | 67.1 | 96.0 | 3.9 | 0.1 |
| 16j | 1 | NaOMe | 75.2 | 99.8 | 0.1 | 0.1 |
| 17k | 1 | NaOH | 73.0 | 97.1 | 2.9 | — |
It is clear that complexes based on small bite angle 1,1-bis(diphenylphosphino)methane ligands are again the most successful (compare run 1 with 2 and 3). Only extremely low activity and poor selectivity is observed for wider bite angle diphosphines. Selectivity is remarkably high for the best systems, up to 99.8% in the case of run 16, even at high conversion. In ethanol to n-butanol coupling, selectivity generally tails off at high conversion for batch reactions, since the increasing concentration of n-butanol facilitates further coupling reactions with this product.4–6 In the case of isobutanol, further Guerbet catalysis is disfavoured due to the difficulty in dehydrogenation to isobutanol.10c The main product observed other than isobutanol, is a small amount (1.8% in run 1) of propanol – the intermediate in isobutanol production. The P–N ligand systems that we recently reported to also be highly efficient and water tolerant catalysts for ethanol to n-butanol catalysis are reasonably successful here (run 4) and with a longer reaction time (20 h) good conversion at similar selectivity can be achieved (run 5). Some loss in activity is observed as the amine group is methylated (runs 6 and 7) but moderate activity is still observed with the fully methylated ligand 6, seemingly ruling out an outer-sphere type mechanism.
Catalyst 1 works well with a variety of bases (runs 8–10), in particular even hydroxide bases give excellent results (runs 9 and 10). Reducing base concentration is to the detriment of performance (runs 11–13), as has been observed in other systems.10 Activity also drops dramatically below 120 °C (run 14), typical for many borrowed hydrogen catalysts. However good activity and selectivity is restored at 150 °C when the reaction time is increased to 20 h (run 15).
Experiments were conducted to investigate whether the catalyst system could be recycled (see ESI† for data and more details). Removal of all volatiles post reaction and adding fresh substrate (ethanol/methanol) gave a 40% drop in activity compared to a virgin run. However, addition of fresh substrate and fresh base at the end of a run allows the catalyst to be recycled three times and still produce isobutanol in good yield. These results suggest that base deactivation may be a deciding factor. Deactivation may also occur due to the formation of water as the reaction proceeds. However, when water was added at the start of a run (0.62 mL, 2 molar equivalents with respect to ethanol) the catalyst was remarkably robust, the same results within error being obtained compared to an absence of initial water (compare runs 9 and 17).
The proposed Guerbet mechanism is supported by the observation of the intermediate propanol as a minor product in reactions. This is further corroborated by a labelling study in which 13CH3OH is used under standard conditions with unlabelled ethanol. The 13C label is observed by NMR spectroscopy to be exclusively in the methyl positions of the isobutanol product, as expected from the proposed series of aldol condensations (see ESI†).
In conclusion, we report an extremely selective and productive homogeneous ruthenium catalyst for the production of the advanced biofuel molecule isobutanol from methanol–ethanol mixtures. Complexes supported by small bite angle disphosphines give the best performance, with preliminary mechanistic studies supportive of a Guerbet-type mechanism.§
Notes and references
- (a) A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484 CrossRef CAS PubMed; (b) M. Guo, W. Song and J. Buhain, Renewable Sustainable Energy Rev., 2015, 42, 712 CrossRef CAS.
- Many examples including: (a) S. Atsumi, A. F. Cann, M. R. Connor, C. R. Shen, K. M. Smith, M. P. Brynildsen, K. J. Y. Chou, T. Hanai and J. C. Liao, Metab. Eng., 2008, 10, 305 CrossRef CAS PubMed; (b) B. G. Harvey and H. A. Meylemans, J. Chem. Technol. Biotechnol., 2011, 86, 2 CrossRef CAS.
- (a) E. M. Green, Curr. Opin. Biotechnol., 2011, 22, 337 CrossRef CAS PubMed; (b) C. Jin, M. Yao, H. Liu, C.-F. Lee and J. Ji, Renewable Sustainable Energy Rev., 2011, 15, 4080 CrossRef CAS.
- G. R. M. Dowson, M. F. Haddow, J. Lee, R. L. Wingad and D. F. Wass, Angew. Chem., Int. Ed., 2013, 52, 9005 ( Angew. Chem. , 2013 , 125 , 9175 ) CrossRef CAS PubMed.
- R. L. Wingad, P. J. Gates, S. T. G. Street and D. F. Wass, ACS Catal., 2015, 5, 5822 CrossRef CAS.
- (a) K.-N. T. Tseng, S. Lin, J. W. Kampf and N. K. Szymczak, Chem. Commun., 2016, 52, 2901 RSC; (b) S. Chakraborty, P. E. Piszel, C. E. Hayes, R. T. Baker and W. D. Jones, J. Am. Chem. Soc., 2015, 137, 14264 CrossRef CAS PubMed; (c) G. Xu, T. Lammens, Q. Liu, X. Wang, L. Dong, A. Caiazzo, N. Ashraf, J. Guan and X. Mu, Green Chem., 2014, 16, 3971 RSC; (d) K. Koda, T. Matsu-ura, Y. Obora and Y. Ishii, Chem. Lett., 2009, 38, 838 CrossRef CAS.
- A. M. Brownstein, Renewable Motor Fuels: The Past, the Present and the Uncertain Future, Butterworth-Heinemann, Oxford, 2014, ch. 5, p. 47 Search PubMed.
- M. Guerbet, C. R. Acad. Sci., 1899, 128, 1002 Search PubMed; M. M. Guerbet, C. R. Acad. Sci., 1909, 149, 129 Search PubMed; S. Veibel and J. I. Nielsen, Tetrahedron, 1967, 23, 1723 CrossRef CAS; A. J. O'Lenick Jr., J. Surfactants Deterg., 2001, 4, 311 CrossRef; D. Gabriëls, W. Y. Hernández, B. Sels, P. Van Der Voort and A. Verberckmoes, Catal. Sci. Technol., 2015, 5, 3876 Search PubMed.
- Reviews of borrowed hydrogen activation of alcohols: (a) M. Haniti, S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555 CrossRef; (b) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS PubMed ; for related ruthenium dehydrogenation-based chemistry, see: ; (c) D. Morton, D. J. Cole-Hamilton, I. D. Utuk, M. Paneque-Sosa and M. Lopez-Poveda, J. Chem. Soc., Dalton Trans., 1989, 489 RSC; (d) M. G. Edwards, R. F. R. Jazzar, B. M. Paine, D. J. Shermer, M. K. Whittlesey, J. M. J. Williams and D. D. Edney, Chem. Commun., 2004, 90 RSC; (e) P. J. Black, M. G. Edwards and J. M. J. Williams, Eur. J. Org. Chem., 2006, 4367 CrossRef CAS.
- (a) W. Ueda, T. Kuwabara, T. Ohshida and Y. Morikawa, J. Chem. Soc., Chem. Commun., 1990, 1558 RSC; (b) W. Ueda, T. Ohshida, T. Kuwabara and Y. Morikawa, Catal. Lett., 1992, 12, 97 CrossRef CAS; (c) C. Carlini, M. Di Girolamo, A. Macinai, M. Marchionna, M. Noviello, A. Maria, R. Galletti and G. Sbrana, J. Mol. Catal., 2003, 200, 137 CrossRef CAS; (d) E. S. Olson, R. K. Sharma and T. R. Aulich, Appl. Biochem. Biotechnol., 2004, 113–116, 913 CrossRef CAS PubMed ; for related Guerbet coupling of methanol and n-propanol see ; (e) C. Carlini, M. Di Girolamo, M. Marchionna, M. Noviello, A. M. R. Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 2002, 184, 273 CrossRef CAS; (f) C. Carlini, M. Di Girolamo, A. Macinai, M. Marchionna, M. Noviello, A. M. R. Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 2003, 204, 721 CrossRef; (g) C. Carlini, A. Macinai, M. Marchionna, M. Noviello, A. M. R. Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 2003, 206, 409 CrossRef CAS; (h) C. Carlini, M. Marchionna, M. Noviello, A. M. Raspolli Galetti, G. Sbrana, F. Basile and A. Vaccari, J. Mol. Catal. A: Chem., 2005, 232, 315 CrossRef ; for methanation of other alcohols see ; (i) J. Sabadie and G. Descotes, Bull. Soc. Chim. Fr., 1983, 253 CAS; (j) Y. Li, H. Li, H. Junge and M. Beller, Chem. Commun., 2014, 50, 14991 RSC; (k) We note also the conversion of synthesis gas to isobutanol, presumably involving related chemistry: K. A. N. Verkerk, B. Jaeger, C.-H. Finkeldei and W. Keim, Appl. Catal., A, 1999, 186, 407 CrossRef CAS.
- Synthesis of complexes; 1: J. Chatt and R. G. Hayter, J. Chem. Soc., 1961, 896 RSC ; 2: M. A. Fox, M. J. E. Harris, S. Heider, V. Pérez-Gregorio, M. E. Zakrzewska, J. D. Farmer, D. S. Yufit, J. A. K. Howard and P. J. Low, J. Organomet. Chem., 2009, 694, 2350 CrossRef CAS ; 3: I. Warad, Z. Al-Othman, S. Al-Resayes, S. S. Al-Deyab and E.-R. Kenaey, Molecules, 2010, 15, 1028 CrossRef PubMed ; 4: L. Saudan, P. Dupau, J. J. Riedhauser and P. Wyss, US. Pat. Appl. Publ., US20100280273 A1 20101104, 2010 CrossRef; 6: R. Morris, A. Habtemariam, Z. Guo, S. Parsons and P. J. Sadler, Inorg. Chim. Acta, 2002, 339, 551 CrossRef . See ESI† for synthesis of complex 5.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c6cc01599a |
‡ When using sodium methoxide as the base the molar ratio of methanol/methoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol is 16.4. ethanol is 16.4. |
| § Renan Cariou, Ian Dobson, Helen Mason, Glenn Sunley and Russell Taylor (all BP) are thanked for useful discussions. BP Biofuels Ltd and the EPSRC are thanked for funding. |
| This journal is © The Royal Society of Chemistry 2016 |