Open Access Article
Open Access ArticleStabilization of a Zn(II) hydrosulfido complex utilizing a hydrogen-bond accepting ligand†
Matthew D.
Hartle
a,
Mayra
Delgado
b,
John D.
Gilbertson
*b and
Michael D.
Pluth
*a
aDepartment of Chemistry and Biochemistry, Materials Science Institute, Institute of Molecular Biology, University of Oregon, Eugene, Oregon 97403, USA. E-mail: pluth@uoregon.edu
bDepartment of Chemistry, Western Washington University, Bellingham, WA 98225, USA. E-mail: John.gilbertson@wwu.edu
First published on 18th May 2016
Abstract
Hydrogen sulfide (H2S) has gained recent attention as an important biological analyte that interacts with bioinorganic targets. Despite this importance, stable H2S or HS− adducts of bioinorganic metal complexes remain rare due to the redox activity of sulfide and its propensity to form insoluble metal sulfides. We report here reversible coordination of HS− to Zn(didpa)Cl2, which is enabled by an intramolecular hydrogen bond between the zinc hydrosulfido product and the pendant tertiary amine of the didpa ligand.
Although historically known for its malodour and toxicity, hydrogen sulfide (H2S) has joined nitric oxide (NO) and carbon monoxide (CO) as a physiologically important gasotransmitter.1 Complicating its reactivity with different bioinorganic targets, H2S has multiple protonation states, participates in complex redox chemistry, and is highly metallophilic.2,3 For example, the high affinity between zinc and sulfide (
 , sphalerite)4 limits generation of stable sulfide-ligated products, but has also been leveraged as a sulfide sequestration strategy in quantification and detection methods.5,6 Consequently, stable zinc hydrosulfido complexes remain rare,7–11 with reported examples including (tris)pyrazolylborate zinc hydrosulfide (TpZnSH) complexes, supported by the steric protection from the Tp ligand.7,8 Complementing the use of steric protection, secondary-coordination sphere interactions have also been used to stabilize metal-sulfide species with other metals, such as iron.12 Additionally, these stabilizing forces are hypothesized to be important in the mechanism of carbonyl sulfide fixation by carbonic anhydrase.13,14
, sphalerite)4 limits generation of stable sulfide-ligated products, but has also been leveraged as a sulfide sequestration strategy in quantification and detection methods.5,6 Consequently, stable zinc hydrosulfido complexes remain rare,7–11 with reported examples including (tris)pyrazolylborate zinc hydrosulfide (TpZnSH) complexes, supported by the steric protection from the Tp ligand.7,8 Complementing the use of steric protection, secondary-coordination sphere interactions have also been used to stabilize metal-sulfide species with other metals, such as iron.12 Additionally, these stabilizing forces are hypothesized to be important in the mechanism of carbonyl sulfide fixation by carbonic anhydrase.13,14
Toward increasing our understanding of the solution state stabilization of metal hydrosulfido complexes, we viewed that a ligand scaffold with a pendant hydrogen-bond (H-bond) acceptor would provide a viable scaffold for Zn–SH stabilization. We noted that the pyridinediimine (PDI) ligand scaffold didpa ([(2,6-iPrC6H3)(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)(N(iPr)2C2H4)(N
CMe)(N(iPr)2C2H4)(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)C5H3N]) has been demonstrated to function as an H-bond donor when the pendant diisopropylamine is protonated and used to stabilize metal halogen hydrogen bonds (MHHBs), with calculated MHHB strengths of ∼6 kcal mol−1 for [Zn(Hdidpa)Cl2][PF6].15 Additionally, the protonated form of the ligand can also function as an H-bond donor to stabilize rare Fe-OH species.16 Although ligands displaying hydrogen-bond donors in the secondary coordination sphere are now frequently used in bioinorganic model complexes,17,18 hydrogen-bond accepting ligands are rare.19–23 On the basis of these properties, we reasoned that the didpa ligand could function as an H-bond acceptor in its neutral form to provide a suitable ligation environment for metal hydrosulfido stabilization.
CMe)C5H3N]) has been demonstrated to function as an H-bond donor when the pendant diisopropylamine is protonated and used to stabilize metal halogen hydrogen bonds (MHHBs), with calculated MHHB strengths of ∼6 kcal mol−1 for [Zn(Hdidpa)Cl2][PF6].15 Additionally, the protonated form of the ligand can also function as an H-bond donor to stabilize rare Fe-OH species.16 Although ligands displaying hydrogen-bond donors in the secondary coordination sphere are now frequently used in bioinorganic model complexes,17,18 hydrogen-bond accepting ligands are rare.19–23 On the basis of these properties, we reasoned that the didpa ligand could function as an H-bond acceptor in its neutral form to provide a suitable ligation environment for metal hydrosulfido stabilization.
Treatment of Zn(didpa)Cl2 (1) with H2S gas failed to produce any reaction, however treatment of 1 with NBu4SH24 in either CH2Cl2 (Fig. 1) or MeCN (Fig. S1, ESI†) resulted in a significant change in the UV-Vis spectrum. In CH2Cl2 the shoulder at 313 nm decreased in intensity with a concomitant increase in absorbance at 280 nm and a well-anchored isosbestic point at 296 nm upon addition of HS−. A Job plot was consistent with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of HS− to 1 (Fig. 1, inset). In addition, titration with HO− resulted in 1
1 binding of HS− to 1 (Fig. 1, inset). In addition, titration with HO− resulted in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding (Fig. S13, ESI†). Based on these results, and because chloride abstraction was not required for HS− binding, we propose that HS− binds to 1 to generate six-coordinate [Zn(didpa)Cl2SH]− (2, Scheme 1). All attempts to grow single crystals suitable for X-ray structural determination of 2 resulted in precipitation of a white amorphous powder after prolonged standing. Supporting the associative formation of a six-coordinate product, titration of 1 with excess Cl− showed similar spectral changes by UV-Vis spectroscopy (Fig. S3 and S4, ESI†), and the Job plot was consistent with 1
1 binding (Fig. S13, ESI†). Based on these results, and because chloride abstraction was not required for HS− binding, we propose that HS− binds to 1 to generate six-coordinate [Zn(didpa)Cl2SH]− (2, Scheme 1). All attempts to grow single crystals suitable for X-ray structural determination of 2 resulted in precipitation of a white amorphous powder after prolonged standing. Supporting the associative formation of a six-coordinate product, titration of 1 with excess Cl− showed similar spectral changes by UV-Vis spectroscopy (Fig. S3 and S4, ESI†), and the Job plot was consistent with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Cl− binding (Fig. S12, ESI†). Attempts to isolate and crystallize the six-coordinate 1
1 Cl− binding (Fig. S12, ESI†). Attempts to isolate and crystallize the six-coordinate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of Cl− and 1 with a range of different Cl− sources (Bu4NCl, Ph4PCl, PPNCl) resulted in simple recrystallization of the starting chloride salt and 1, presumably due to the reversibility of the process.
1 adduct of Cl− and 1 with a range of different Cl− sources (Bu4NCl, Ph4PCl, PPNCl) resulted in simple recrystallization of the starting chloride salt and 1, presumably due to the reversibility of the process.
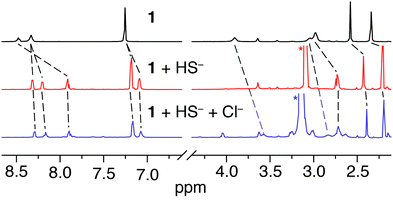
To gain further insights into the HS− binding, we used 1H NMR spectroscopy to investigate the stability of 2 and the role of hydrogen-bonding in the complex. Treatment of 1 with 1.5 equiv. of NBu4SH in CD3CN resulted in significant changes in the 1H NMR spectrum. The resultant spectrum did not match that of the free ligand,15 suggesting that addition of HS− does not remove Zn from the didpa ligand (Fig. 2). Upon HS− addition, the pyridine and other aryl resonances of 1 shifted significantly in a pattern consistent with loss of free rotation of the 2,6-diisopropylphenyl group of the ligand. Similarly, the isopropyl resonance at 1.00 ppm, corresponding to the isopropyl methyl groups of the pendant amine, shifts downfield and bifurcates. Each of these spectral changes indicated a significant change in the primary coordination sphere of the zinc and are consistent with formation of a six-coordinate complex in which the Zn–SH moiety is hydrogen bonded to the pendant diisopropylamine (Scheme 1).
 | ||
| Fig. 2 1H NMR spectra of 1 (11.8 mM in CD3CN) before (top, black) and after (bottom, red) addition of 1.5 equiv. of NBu4SH. Peaks denoted with a (*) correspond to the NBu4+ counterion. | ||
Low temperature 1H NMR investigations provided additional information about the structural flexibility of the scaffold and the presence of the coordinated hydrosulfide. Although we were unable to observe an appreciable signal corresponding to the coordinated HS− at room temperature, we expected that lowering the temperature would not only enable HS− observation, but also lock the conformation of the ethylene backbone of the ligand. Consistent with these expectations, cooling to −35 °C in CD3CN resulted in the appearance of a new resonance at 11.05 ppm, matching the general chemical shift expected for partial protonation of the nitrogen as an H-bond acceptor (Fig. 3).25 For comparison, the 1H NMR spectrum of the previously reported [Zn(Hdidpa)Cl2][PF6] displays an N–H resonance at 8.50 ppm in CD2Cl2 due to the protonated diisopropylamine of the didpa ligand acting as an H-bond donor and forming an intramolecular MHHB with a chloride ligand.15 The downfield shift observed for 2 is consistent with the diisopropylamine acting as an H-bond acceptor, likely accepting a hydrogen bond from the Zn-ligated SH moiety.26
 | ||
| Fig. 3 Variable temperature 1H NMR spectra of 11.4 mM Zn(didpa)Cl2, and 1.5 equiv. NBu4SH in CD3CN. Cooling to −35 °C results in sharpening and an upfield shift of the broad SH peak to 11.05 ppm. See Fig. S9 (ESI†) for an expanded spectrum of the SH peak. | ||
Additionally, we observed that the broad peak centered at 3.6 ppm corresponding to the ethylene bridge decoalesced upon cooling. The presence of the internal hydrogen bond between the sulfhydryl group and the tertiary amine significantly limits the flexibility of the secondary coordination sphere, resulting in a sharpening in the signal produced by the ethylene linker, methine protons, and methyl groups (Fig. S8, ESI†). Supporting the presence of an intramolecular SH hydrogen bond, a 1H NOESY experiment at −35 °C revealed cross peaks between the SH and the ethylene, methine, and methyl signals of the tertiary amine linker (Fig. S5, ESI†).
Although the N⋯HS interaction was not observed by solution state FTIR, we note that the N–H stretch involved in hydrogen bonding in the [Zn(Hdidpa)Cl2][PF6] system is also absent in solution FTIR spectra.15 The N–H stretch in the N⋯HS moiety is likely severely broadened or obscured, which is common in many hydrogen bonding systems.25
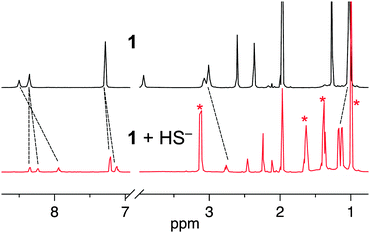
To demonstrate the reversibility of 2, and to provide evidence for the formation of a six-coordinate complex, we performed a displacement experiment by 1H NMR. Treatment of 1 with 1.5 equiv. of NBu4SH resulted in the formation of 2 with characteristic shifts in the aryl peaks, and broad resonance at 3.6 ppm (Fig. 4). Upon addition of 20 equiv. of NBu4Cl, the aryl protons maintained the same configuration; however, the broad absorbance at 3.6 ppm resolved, characteristic of the Cl− adduct of 1 (Fig. S6, ESI†).27
To further determine whether the internal hydrogen-bond was necessary for stabilization of the Zn–SH product, we also titrated a Zn-pyridinediimine compound which does not possess the pendant amine hydrogen-bond acceptor (Scheme 2). Upon addition of 0.5 equiv. of HS− to a solution of Zn(iPrPDI)Cl2 (3) (iPrPDI = 2,6-(2,6-iPr2C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2C5H3N) a white precipitate (ZnS) was immediately observed, along with a significant change in the NMR spectrum. The shifts in the NMR spectrum and formation of precipitate continued until 1 equiv. of HS− was added, after which no more changes were observed (Fig. S11, ESI†). The resultant solid was isolated, washed with acetonitrile to remove any excess free sulfide, then acidified and subjected to the methylene blue assay, which provided a positive response for acid-labile sulfur and was consistent with ZnS formation.28 These data illustrate the importance of the hydrogen-bond acceptor in the stabilization of the zinc-sulfido complex, as the pendant amine in 1 is vital in this system.
CMe)2C5H3N) a white precipitate (ZnS) was immediately observed, along with a significant change in the NMR spectrum. The shifts in the NMR spectrum and formation of precipitate continued until 1 equiv. of HS− was added, after which no more changes were observed (Fig. S11, ESI†). The resultant solid was isolated, washed with acetonitrile to remove any excess free sulfide, then acidified and subjected to the methylene blue assay, which provided a positive response for acid-labile sulfur and was consistent with ZnS formation.28 These data illustrate the importance of the hydrogen-bond acceptor in the stabilization of the zinc-sulfido complex, as the pendant amine in 1 is vital in this system.
 | ||
| Scheme 2 Reaction of 3 with NBu4SH shows decomposition, demonstrating the importance of the H-bond acceptor in 2. | ||
In conclusion, we have shown the stabilization of a rare synthetic zinc hydrosulfide complex stabilized by a hydrogen bond accepting ligand. Removal of the hydrogen bonding ability of the ligand resulted in decomposition and ZnS precipitation, highlighting the importance of the second coordination sphere in stabilizing the zinc hydrosulfido adduct.
This work was supported by CAREER Awards from the National Science Foundation (CHE-1454747 to MDP, CHE-1255570 to JDG) and the Sloan Foundation (MDP). The NMR facilities at the University of Oregon are supported by NSF/ARRA (CHE-0923589).
Notes and references
- R. Wang, Physiol. Rev., 2012, 92, 791–896 CrossRef CAS PubMed.
- M. D. Hartle, S. K. Sommer, S. R. Dietrich and M. D. Pluth, Inorg. Chem., 2014, 53, 7800–7802 CrossRef CAS PubMed.
- C. C. Tsou, W. C. Chiu, C. H. Ke, J. C. Tsai, Y. M. Wang, M. H. Chiang and W. F. Liaw, J. Am. Chem. Soc., 2014, 136, 9424–9433 CrossRef CAS PubMed.
- P. Patnaik, Handbook of Inorganic Chemicals, McGraw-Hill, New York, 2002 Search PubMed.
- J. K. Fogo and M. Popowsky, Anal. Chem., 1949, 21, 732–734 CrossRef CAS.
- E. Galardon, A. Tomas, P. Roussel and I. Artaud, Dalton Trans., 2009, 9126–9130 RSC.
- M. Rombach and H. Vahrenkamp, Inorg. Chem., 2001, 40, 6144–6150 CrossRef CAS PubMed.
- M. Ruf and H. Vahrenkamp, Inorg. Chem., 1996, 35, 6571–6578 CrossRef CAS PubMed.
- M. M. Morlok, K. E. Janak, G. Zhu, D. A. Quarless and G. Parkin, J. Am. Chem. Soc., 2005, 127, 14039–14050 CrossRef CAS PubMed.
- N. G. Spiropulos, E. A. Standley, I. R. Shaw, B. L. Ingalls, B. Diebels, S. V. Krawczyk, B. F. Gherman, A. M. Arif and E. C. Brown, Inorg. Chim. Acta, 2012, 386, 83–92 CrossRef CAS.
- S. Kuwata and M. Hidai, Coord. Chem. Rev., 2001, 213, 211–305 CrossRef CAS.
- E. Galardon, T. Roger, P. Deschamps, P. Roussel, A. Tomas and I. Artaud, Inorg. Chem., 2012, 51, 10068–10070 CrossRef CAS PubMed.
- J. Notni, S. Schenk, G. Protoschill-Krebs, J. Kesselmeier and E. Anders, ChemBioChem, 2007, 8, 530–536 CrossRef CAS PubMed.
- F. Namuswe and J. M. Berg, J. Inorg. Biochem., 2012, 111, 146–149 CrossRef CAS PubMed.
- M. Delgado, S. K. Sommer, S. P. Swanson, R. F. Berger, T. Seda, L. N. Zakharov and J. D. Gilbertson, Inorg. Chem., 2015, 54, 7239–7248 CrossRef CAS PubMed.
- A. J. Kendall, L. N. Zakharov and J. D. Gilbertson, Inorg. Chem., 2010, 49, 8656–8658 CrossRef CAS PubMed.
- A. S. Borovik, Acc. Chem. Res., 2005, 38, 54–61 CrossRef CAS PubMed.
- S. A. Cook and A. S. Borovik, Acc. Chem. Res., 2015, 48, 2407–2414 CrossRef CAS PubMed.
- Y. J. Park, N. S. Sickerman, J. W. Ziller and A. S. Borovik, Chem. Commun., 2010, 46, 2584–2586 RSC.
- N. S. Sickerman, Y. J. Park, G. K. Ng, J. E. Bates, M. Hilkert, J. W. Ziller, F. Furche and A. S. Borovik, Dalton Trans., 2012, 41, 4358–4364 RSC.
- Z. Shirin and C. J. Carrano, Polyhedron, 2004, 23, 239–244 CrossRef CAS.
- C. M. Moore and N. K. Szymczak, Dalton Trans., 2012, 41, 7886–7889 RSC.
- E. M. Matson, J. A. Bertke and A. R. Fout, Inorg. Chem., 2014, 53, 4450–4458 CrossRef CAS PubMed.
- M. D. Hartle, D. J. Meininger, L. N. Zakharov, Z. J. Tonzetich and M. D. Pluth, Dalton Trans., 2015, 44, 19782–19785 RSC.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48–76 CrossRef CAS.
- B. Brzeninski and G. Zundel, J. Mol. Struct., 1982, 84, 205–211 CrossRef.
- Based on the number of equivalents of HS− required to displace the bound Cl−, we estimate that the binding affinity of HS− for Zn(didpa)Cl2 is similar and not more than 10× greater than that of Cl−. See Fig. S3 (ESI†).
- X. Shen, C. B. Pattillo, S. Pardue, S. C. Bir, R. Wang and C. G. Kevil, Free Radical Biol. Med., 2011, 50, 1021–1031 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional spectroscopic and NMR data. See DOI: 10.1039/c6cc01373b |
| This journal is © The Royal Society of Chemistry 2016 |