 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A water splitting system using an organo-photocathode and titanium dioxide photoanode capable of bias-free H2 and O2 evolution†
Toshiyuki
Abe
*a,
Katsuma
Fukui
a,
Yuto
Kawai
a,
Keiji
Nagai
b and
Hideki
Kato
c
aDepartment of Frontier Materials Chemistry, Graduate School of Science and Technology, Hirosaki University, 3 Bunkyo-cho, Hirosaki 036-8561, Japan. E-mail: tabe@hirosaki-u.ac.jp
bChemical Resources Laboratory, Tokyo Institute of Technology, Suzukake-dai, Midori-ku, Yokohama 226-8503, Japan
cInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
First published on 23rd May 2016
Abstract
This study examined a water-splitting system comprising a TiO2 photoanode and an organo-photocathode consisting of a p–n bilayer. Stoichiometric decomposition of water into H2 and O2 successfully occurred at bias voltages lower than the theoretical value (i.e. 1.23 V). Compared to the conventional TiO2 and Pt systems, the proposed water-splitting system demonstrated water splitting without any externally applied bias.
For establishing a sustainable society, solar hydrogen has been considered to be a potentially clean energy substitute for fossil fuels. For acquiring solar hydrogen, water splitting has been actively investigated using both photoelectrochemical and photocatalytic approaches.1–11 Since the discovery of photoelectrochemical water splitting by Honda and Fujishima,12 TiO2 has been recognised as one of the most efficient photoanodes for the evolution of O2 from water. Various types of structures with TiO2 have been fabricated based on novel approaches.13–15 However, when TiO2 is used as a photoanode along with a Pt counter electrode (cf. the corresponding system is abbreviated as TiO2–Pt), the overall splitting of water into O2 and H2 occurs only upon the application of an external chemical or electrochemical bias.13–17 This is because of the close potential of the conduction band (corresponding to the magnitude of reducing power) to the reduction potential of H+ into H2.
Photoelectrochemical and photocatalytic systems featuring organic semiconductors and a p–n bilayer have been investigated in our previous studies.18–23 In some instances, photocatalytic reactions via organic p–n bilayers respond to the entire visible-light energy for the oxidative decomposition of organoamine,19,20 alcohol,21 aldehyde21 and hydrazine.22,23 In addition, when an organobilayer of phthalocyanine (H2Pc, p type) and fullerene (C60, n type) was employed as a photocathode in a three-electrode system,24 the C60 surface modified by Pt induced the reduction of H+ into H2 at applied potentials that were more positive than the formal potential of H+/H2. The detailed characteristics of C60 for producing H2 were clarified based on two types of in situ spectroelectrochemistry (i.e. VIS–NIR and Raman).24 Recently, more efficient H2 production was found to occur by the replacement of H2Pc by zinc phthalocyanine (ZnPc, p type).25
In the present study, a photoelectrochemical water-splitting system was studied, where TiO2 (photoanode) and a ZnPc/C60 bilayer modified by Pt (photocathode, vide supra) were simultaneously employed for water oxidation and H+ reduction, respectively (Scheme 1). The stoichiometric decomposition of water into H2 and O2 was found to occur upon application of bias voltages <1.23 V. Moreover, this study demonstrated that photocatalytic water decomposition (i.e. bias-free water splitting) occurs successfully in a system featuring an organo-photocathode, making it distinct from a conventional TiO2–Pt system (vide supra).
The ZnPc/C60 bilayer was prepared by vapour deposition (pressure, <1.0 × 10−3 Pa; deposition speed, ca. 0.03 nm s−1) using an indium-tin-oxide (ITO)-coated glass plate as the base material.25 The organic p–n bilayer was composed of ZnPc coated on ITO and C60 coated on top of the ZnPc layer; moreover, a Pt co-catalyst was photocathodically deposited onto the C60 surface of the bilayer (the resulting device is abbreviated as ITO/ZnPc/C60–Pt). A disk-like form of TiO2 (rutile) was utilised as the photoanode, which was reductively treated under a hydrogen flow at 773 K for 2 h prior to use. In order to achieve an ohmic contact, Ga–In was applied to the back side of the TiO2. As represented in Scheme 1, a cell made up of twin compartments separated by a salt bridge was utilised for the water-splitting studies. All studies were performed under an Ar atmosphere in an aqueous H3PO4 solution (pH = 2). Other experimental details are provided in the ESI.†
The voltammetric characteristics of photoelectrodes employed were examined in a three-electrode system (see Scheme S1 and Fig. S1 (ESI†)). When using TiO2 as a photoanode, a photocurrent due to the oxidation of H2O was observed at potentials more positive than 0 V (vs. Ag/AgCl (sat.)). The voltammogram measured for ITO/ZnPc/C60–Pt is shown in Fig. S1 (ESI†).25 The CV data revealed that both the photoanodic and photocathodic currents are generated in the same potential window. In other words, the water-splitting system of TiO2 and ITO/ZnPc/C60–Pt may produce H2 and O2 without any bias voltage.
A water-splitting study was conducted with and without applied bias voltages (Scheme 1). The stoichiometric decomposition of water into H2 and O2 was found to occur at bias voltages less than 1.23 V (the theoretical voltage for water splitting). Fig. 1(a) shows the relationship between the amounts of H2 and O2 evolved and the respective applied voltages. The H2 and O2 amounts increased when a large bias was applied to the system; however, the evolved amounts gently increased, particularly at voltages larger than 0.25 V. The bias-free H2 and O2 evolution occurred stoichiometrically (cf. detailed data are also shown in the following figures). Based on the results shown in Fig. 1(a), the light to hydrogen conversion efficiency (η, ESI†) was estimated with respect to the applied voltages. As shown in Fig. 1(b), efficient water splitting occurred at a small bias voltage of 0.25 V with ca. 0.1%. The results of Fig. 1(a) and (b) can be interpreted as follows. When a bias voltage is applied to the system, efficient charge separation can occur along with an efficient charge transfer between the photoanode and the photocathode, which can involve the suppression of carrier recombination. In such an occasion, the amounts of H2 and O2 may essentially increase. However, it appeared that a large bias of >0.25 V cannot cause the proportional formation of H2 and O2 due to the presence of carriers sufficient to induce water splitting at those voltages. For example, the band bending for water oxidation at TiO2 can sufficiently occur to be almost independent of carrier formation with respect to applied voltages. Therefore, the application of a large bias voltage to a water splitting system can only lead to a gentle enhancement of kinetics for H2 and O2 formation, thus resulting in decreasing efficiency (η). Such a phenomenon was also exhibited in other systems of water splitting.3,26–28
 | ||
| Fig. 1 Relationships of (a) the evolved H2 and O2 amounts and (b) η values with applied voltages. This study was conducted in the two-electrode system depicted in Scheme 1. The amounts of H2 and O2 evolved with a 2h-irradiation time are represented. Faradaic efficiencies for H2 and O2 evolution were >90% and >80%, respectively (ESI†). Photoanode, TiO2 (geometrical area, 0.5 cm2); photocathode, ITO/ZnPc(75 nm)/C60(125 nm)–Pt (geometrical area, 1 cm2); electrolyte, H3PO4 solution (pH = 2); light intensity (for photoanode), ca. 70 mW cm−2; light intensity (for photocathode), ca. 90 mW cm−2. | ||
A prolonged study was conducted to test the durability of the present system. The linear relationships between the amounts of H2 and O2 evolved and the irradiation time, demonstrating the stable and durable performance for water splitting with and without bias voltages, are shown in Fig. 2.
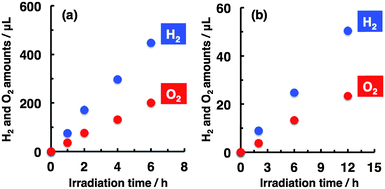 | ||
| Fig. 2 Relationships between the H2 and O2 amounts and irradiation time ((a) 0.7 V; (b) 0 V, i.e. no-biased condition). Experimental conditions were the same as those used in Fig. 1, except that the irradiation time was changed. | ||
Control experiments conducted in the presence of methanol (electron donor) or Ag+ (electron acceptor) were compared with a typical result from the present system, as shown in Table 1. Irrespective of the presence of methanol, the amount of H2 evolved was relatively constant (Table 1, entries 1 and 2); however, in the presence of an Ag+ acceptor, the amount of evolved O2 increased (Table 1, entries 1 and 3). Therefore, these results may suggest that the kinetics of the present system (Table 1, entry 1) is dominated by H2 formation.
| System | H2 evolved/μL | O2 evolved/μL | Note |
|---|---|---|---|
a Bias voltage of 0.7 V was applied to the system under experimental conditions similar to those in Fig. 1.
b Data obtained from Fig. 1.
c A methanol solution (methanol/aqueous H3PO4 solution (v/v) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 2) was used.
d An aqueous solution of AgNO3 (5 mM, pH = 2) was employed. 1, pH = 2) was used.
d An aqueous solution of AgNO3 (5 mM, pH = 2) was employed.
|
|||
| Entry 1b | 171 | 76.2 | No control system |
| Entry 2c | 176 | — | In the presence of methanol |
| Entry 3d | — | 101 | In the presence of Ag+ |
The evolution of H2 and O2 in the conventional TiO2–Pt system was examined for use as a reference system. Similar to previous results (vide supra), Fig. S2 (ESI†) demonstrates that the TiO2–Pt system needs an applied bias voltage for achieving water splitting. A comparison between the present and conventional systems is represented in Table 2. The amounts of H2 and O2 originating from water splitting are greater in the TiO2–ITO/ZnPc/C60–Pt system compared to the TiO2–Pt system. These results indicate that rate-limiting H2 evolution becomes more efficient when employing ITO/ZnPc/C60–Pt instead of a Pt counter as the cathode.
| System | Photoanode | Cathode | H2 evolved/μL | O2 evolved/μL |
|---|---|---|---|---|
| a Bias voltage of 0.7 V was applied to the system, and other experimental conditions were similar to those of Fig. 1. b Data obtained from Fig. 1. c Instead of ITO/ZnPc/C60–Pt, a Pt wire was employed as the cathode. Data listed in this table can also be seen in Fig. S2 (ESI). | ||||
| Entry 1b | TiO2 | ITO/ZnPc/C60–Pt | 171 | 76.2 |
| Entry 2c | TiO2 | Pt wire | 86.7 | 44.0 |
The individual reactivity of the photoelectrodes employed in TiO2–ITO/ZnPc/C60–Pt has been previously clarified: O2 and H2 evolution can occur by the oxidising and reducing power produced at the TiO2 photoanode13–17 and the ITO/ZnPc/C60–Pt photocathode,24,25 respectively. Distinct from the conventional TiO2–Pt system, the electrons photogenerated at TiO2 cannot directly participate in the reduction of H+ into H2; however, they can contribute to the regeneration of the pristine ZnPc through electron transfer via an external circuit (Scheme 1). Action spectra for photocurrents generated at TiO2 and ITO/ZnPc/C60–Pt are shown in Fig. S3 (ESI†), confirming that TiO2 (rutile) can induce O2 evolution based on bandgap excitation (3.06 eV in rutile TiO2).29,30 In addition, we have previously reported that the entire visible-light energy range is available for H2 evolution occurring at an ITO/ZnPc/C60–Pt electrode.25
In summary, the photoelectrochemical splitting of water was demonstrated in a TiO2–ITO/ZnPc/C60–Pt system featuring an organo-photocathode with a TiO2 photoanode, and bias-free decomposition of water into H2 and O2 was also found to occur. Based on the resulting action spectral characteristics (ESI†), the full region of visible light energy (i.e. <750 nm in wavelength) was available for H2 evolution at the organo-photocathode. This implies that efficient water splitting can occur when simultaneously utilising a photoanode, responsive to visible light, for O2 evolution. Thus, this work exhibited the effectiveness of using an organic p–n bilayer for a water-splitting system. The application of two types of materials for water splitting has merits in terms of the harvesting of solar energy and individual generation of oxidising and reducing power for O2 and H2 evolution, respectively. This is similar to the Z-scheme type photocatalytic water splitting.31–34 Novel and efficient photoelectrodes for H2 and O2 evolution need to be developed for both organic and inorganic semiconductors so that a breakthrough in establishing a practical system for water splitting can be found.
This work was partly supported by a grant from the Murata Science Foundation (T. A.) and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” and a Grant-in-Aid for Scientific Research on Innovative Areas (T. A. and H. K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Q. Yu, X. Meng, T. Wang, P. Li and J. Ye, Adv. Funct. Mater., 2015, 25, 2686 CrossRef CAS.
- J. Wang, P. Zhang, X. Song and L. Gao, RSC Adv., 2015, 5, 1220 RSC.
- I. Fujimoto, N. Wang, R. Saito, Y. Miseki, T. Gunji and K. Sayama, Int. J. Hydrogen Energy, 2014, 39, 2454 CrossRef CAS.
- H. Higashi, K. Domen and R. Abe, J. Am. Chem. Soc., 2013, 135, 10238 CrossRef PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990 CrossRef CAS PubMed.
- T. Oshima, D. Lu, O. Ishitani and K. Maeda, Angew. Chem., Int. Ed., 2015, 54, 2698 CrossRef CAS PubMed.
- T. Takata, C. Pan, M. Nakabayashi, N. Shibata and K. Domen, J. Am. Chem. Soc., 2015, 137, 9627 CrossRef CAS PubMed.
- C. S. Quintans, H. Kato, M. Kobayashi, H. Kaga, A. Iwase, A. Kudo and M. Kakihana, J. Mater. Chem. A, 2015, 3, 14239 CAS.
- T.-F. Yeh, C.-Y. Teng, S.-J. Chen and H. Teng, Adv. Mater., 2014, 26, 3297 CrossRef CAS PubMed.
- F. Fresno, R. Portela, S. Suárez and J. M. Coronado, J. Mater. Chem. A, 2014, 2, 2863 CAS.
- Z. Li, W. Luo, M. Zhang, J. Feng and Z. Zou, Energy Environ. Sci., 2013, 6, 347 CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- L. N. Quan, Y. H. Jang, K. A. Stoerzinger, K. J. May, Y. J. Jang, S. T. Kochuveedu, Y. Shao-Horn and D. H. Kim, Phys. Chem. Chem. Phys., 2014, 16, 9023 RSC.
- H. Li, J. Chen, Z. Xia and J. Xing, J. Mater. Chem. A, 2015, 3, 699 CAS.
- S. Hernández, D. Hidalgo, A. Sacco, A. Chiodoni, A. Lamberti, V. Cauda, E. Tresso and G. Saracco, Phys. Chem. Chem. Phys., 2015, 17, 7775 RSC.
- M. S. Wrighton, D. S. Ginley, P. T. Wolczanski, A. B. Ellis, D. L. Morse and A. Linz, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 1518 CrossRef CAS.
- A. Fujishima, K. Kohayakawa and K. Honda, J. Electrochem. Soc., 1975, 122, 1487 CrossRef CAS.
- T. Abe, K. Nagai, S. Kabutomori, M. Kaneko, A. Tajiri and T. Norimatsu, Angew. Chem., Int. Ed., 2006, 45, 2778 CrossRef CAS PubMed.
- K. Nagai, T. Abe, Y. Kaneyasu, Y. Yasuda, I. Kimishima, T. Iyoda and H. Imaya, ChemSusChem, 2011, 4, 727 CrossRef CAS PubMed.
- K. Nagai, Y. Yasuda, T. Iyoda and T. Abe, ACS Sustainable Chem. Eng., 2013, 1, 1033 CrossRef CAS.
- S. Zhang, P. Arunachalam, T. Abe, T. Iyoda and K. Nagai, J. Photochem. Photobiol., A, 2012, 244, 18 CrossRef CAS.
- T. Abe, N. Taira, Y. Tanno, Y. Kikuchi and K. Nagai, Chem. Commun., 2014, 50, 1950 RSC.
- T. Abe, Y. Tanno, N. Taira and K. Nagai, RSC Adv., 2015, 5, 46325 RSC.
- T. Abe, S. Tobinai, N. Taira, J. Chiba, T. Itoh and K. Nagai, J. Phys. Chem. C, 2011, 115, 7701 CAS.
- T. Abe, Y. Hiyama, K. Fukui, K. Sahashi and K. Nagai, Int. J. Hydrogen Energy, 2015, 40, 9165 CrossRef CAS.
- J. Akikusa and S. U. M. Khan, Int. J. Hydrogen Energy, 2002, 27, 863 CrossRef CAS.
- M. Radecka, M. Rekas, A. Trenczek-Zajac and K. Zakrzewska, J. Power Sources, 2008, 181, 46 CrossRef CAS.
- M. Moriya, T. Minegishi, H. Kumagai, M. Katayama, J. Kubota and K. Domen, J. Am. Chem. Soc., 2013, 135, 3733 CrossRef CAS PubMed.
- J. F. McAleer and L. M. Peter, Faraday Discuss., 1980, 70, 67 RSC.
- A. Goossens, Surf. Sci., 1997, 371, 390 CrossRef CAS.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, J. Photochem. Photobiol., A, 2002, 148, 71 CrossRef CAS.
- R. Abe, M. Higashi and K. Domen, ChemSusChem, 2011, 4, 228 CrossRef CAS PubMed.
- S. Hara, M. Yoshimizu, S. Tanigawa, L. Ni, B. Ohtani and H. Irie, J. Phys. Chem. C, 2012, 116, 17458 CAS.
- H. Kato, Y. Sasaki, N. Shirakura and A. Kudo, J. Mater. Chem. A, 2013, 1, 12327 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including calculation procedure for some types of efficiencies, both cyclic voltammograms and action spectra for photocurrents measured at photoelectrodes employed, and data for the reference system of a TiO2 photoanode and a Pt wire. See DOI: 10.1039/c6cc01225f |
| This journal is © The Royal Society of Chemistry 2016 |

