Open Access Article
Open Access ArticleSucrose-fueled, energy dissipative, transient formation of molecular hydrogels mediated by yeast activity†
César A.
Angulo-Pachón
and
Juan F.
Miravet
*
Departament de Química Inorgánica i Orgànica, Universitat Jaume I, Avda. Sos Baynat s/n, 12071 Castelló, Spain. E-mail: miravet@uji.es
First published on 14th March 2016
Abstract
A biologically mediated, energy dissipative, reversible formation of fibrillar networks is reported. The process of gelation is linked to sucrose-fueled production of CO2 by baker’s yeast (Saccharomyces cerevisiae). Continuous fueling of the system is required to maintain the self-assembled fibrillar network.
A dissipative system is a thermodynamically open system which converts usable energy into non-recoverable forms of work. Such a system is operating out of equilibrium and exchanges energy and/or matter with the environment.1 A dissipative structure is an organized non-equilibrium state of matter created and maintained due to dissipative processes.2 Dissipative structures grow more complex by exporting, or dissipating, entropy into their surroundings.3 The relevance of dissipative chemical systems in life processes was highlighted by the pioneering work of Prigogine.4 Remarkable cases of dissipative chemical systems at the biological level include the formation of fibrillar networks which are essential to organize and rearrange the interior of the cell. For example, actin fibers are fundamental to the structure of the cell. Their dynamic, out-of-equilibrium, transient nature is linked to the so-called “actin cycle”, which couples ATP hydrolysis (fuel) to actin polymerization.5 Microtubules are also involved in maintaining the structure of the cell, forming the cytoskeleton. They are formed by reversible, out-of-equilibrium self-assembly of tubulins fueled by the hydrolysis of GTP.6 Trying to emulate these biological systems is challenging, and a few studies in this regard have emerged in recent years. In particular, molecular hydrogels are of interest because they are formed by self-assembled fibrillar networks. The attention paid to these type of materials has grown significantly in recent years, as a result of their applicability as new soft materials in areas such as molecular electronics, controlled release, or catalysis among others.7–10 Of particular relevance is the application of molecular hydrogels in biomedicine, in particular in relation to tissue engineering.11,12 Van Esch et al. reported transient molecular hydrogels, namely, self-assembled fibrillar networks whose disassembly is linked to energy dissipation by means of ester hydrolysis.13,14 A related system forms transient hydrogels from peptide amphiphiles regulated by enzymatic ester formation and hydrolysis.15 Ulijn et al. have described transient hydrogels formed by tripeptides. In this case, gel formation is fueled by the addition of aspartame, which forms transient tripeptides with quimiotripsine as catalyst. In this system the same enzyme hydrolyzes the tripeptide, dissipating the energy and disassembling the gel.16 Dissipative self-assembly of a membrane transport system has been studied recently fueled by addition of a thioester, being energy dissipated by the intramolecular reaction of the channel forming species, affording caprolactam as waste.17 In another example, transient hydrogel formation using peptides was finely time-programmed using the enzymatic hydrolysis of urea, which resulted in an increase of the pH of the medium and gel disassembly.18
Taking advantage of the reversible character of molecular gels and their stimuli-responsiveness,13 here we report on new molecular hydrogels whose formation/disassembly is regulated by the presence of sucrose as fuel and CO2 release as a dissipative process (Scheme 1).
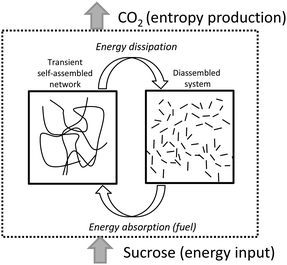 | ||
| Scheme 1 Schematic representation of the transient formation of fibrillar networks fueled by sucrose. Energy dissipation is linked to CO2 release. | ||
The system uses baker’s yeast (Saccharomyces cerevisiae) as a key intermediate for the conversion of sucrose into CO2, constituting a first example of reversible fibrillization in aqueous media linked to biological activity (Scheme 2).
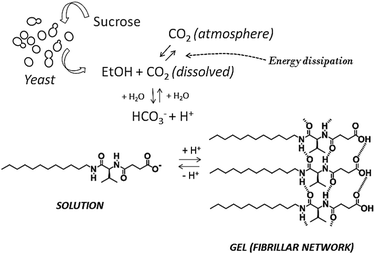 | ||
| Scheme 2 Schematic representation of the network of processes responsible for transient gel formation. | ||
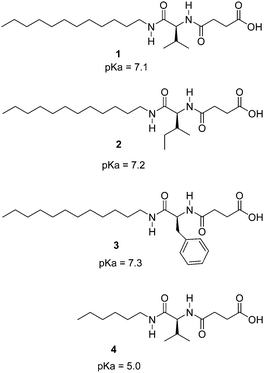
The hydrogelators described in Chart 1 were easily prepared on a gram scale from amino acids by N-acylation with succinic anhydride and amide formation with dodecylamine or hexylamine. The carboxylic acid moiety was introduced to achieve pH sensitivity. Interestingly, initial assays of biocompatibility with the brine shrimp test19 showed that these compounds display null toxicity at the concentrations used in our experiments. These compounds are related to previous hydrogelators obtained by N-acylation with fatty acids of α-amino acids,20 but significant structural differences are present including an additional amide unit. Compounds 1–3 showed remarkable hydrogelation capabilities, forming gels in distilled water upon gentle heating until complete solubilization followed by resting at room temperature.
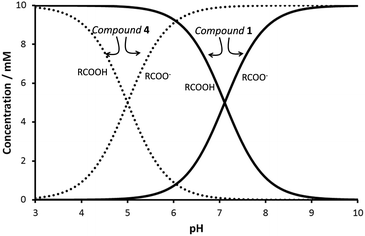
Minimum gelator concentration values required for gelation were 0.2, 0.5 and 1.5% w/w for the phenylalanine, valine and isoleucine derivatives respectively. Compound 4 was not a gelator as a result of the short hydrocarbon chain which most likely does not provide sufficient hydrophobic interactions to form gels in water. The ionic forms of compounds 1–3 (carboxylates) are water soluble, being the neutral species responsible for gelation. In order to study the feasibility of hydrogel formation triggered by pH changes, the pKa of the gelators was assessed. Potentiometric titration of the hydrogelators showed a very remarkable pKa shift of the carboxylic acid unit from the expected value of ca. 5 to ca. 7 (see Chart 1 for pKa values). This decrease in the acidity of the carboxylic acid unit must be ascribed to the formation of the fibrillar network of the hydrogel.21 As seen in Fig. 1, the pH-range of stability of the neutral, gel-forming species is considerably shifted upon going from compound 4 to 1. Therefore compounds 1–3 can form gels around neutral pH values.
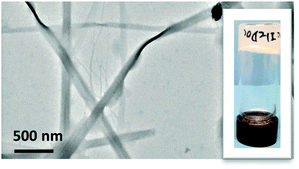
Indeed, hydrogels were readily prepared by maintaining the hydrogelator dissolved in aqueous potassium carbonate for a few hours under a CO2 atmosphere (see pictures of the gels in Fig. 2). Under these conditions, the pH shifted from ca. 12 to ca. 8.
Electron microscopy revealed that the hydrogels prepared in this way consist of elongated fiber-like objects, as commonly observed in molecular gels (Fig. 2). Additionally, X-ray powder diffraction of xerogels indicate some crystalline order in the fibers, resulting in wide diffraction peaks for angles corresponding to distances of ca. 40 ångströms, which correspond to fully extended molecules (see ESI†).
Hydrogelation was linked to CO2 production associated with the activity of baker’s yeast. This type of yeast is used extensively in food and wine production. Additionally, its use in organic transformations has been widely explored.22 In our experiment, sucrose was used to fuel a system composed of an aqueous dispersion of baker’s yeast in a solution.
The pH of the initial suspension was shifted to 10 by the addition of potassium carbonate and the system was sealed with a screw cap. The activity of the yeast transforms sucrose into CO2 and ethanol, neutralizing the medium and therefore driving the system towards gel formation. In a typical experiment, after ca. 2 hours a gel with entrapped yeast was formed with a final pH of ca. 8 (Fig. 3).
If the system is left open to the air, a solution is formed as a result of CO2 elimination. Importantly, adding additional sucrose permitted regeneration of the gel. This gel–solution–gel cycle could be performed at least 5 times.
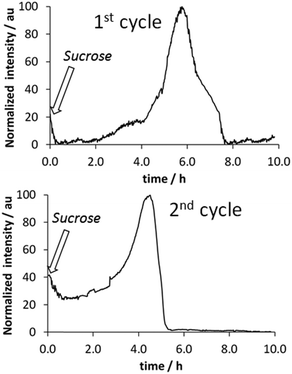
Fluorescence measurements were found to be a convenient way to monitor fiber formation in the case of compound 3, a derivative of phenylalanine. The fluorescence of the phenyl moiety was heavily enhanced as a result of fiber formation21 and therefore a sample containing the gelator dissolved in basic medium and the yeast was studied. Upon addition of sucrose the system was sealed with a polystyrene foam cap. Polystyrene is known to be permeable to CO2,23 and therefore in the studied system the production of CO2 and its slow leakage take place simultaneously. As reflected in Fig. 4, after ca. 2 h, the onset of fibrillization is observed, reaching a maximum after ca. 5 h. Then, the transitory gel is disassembled due to the consumption of the sucrose fuel. Further addition of sucrose gave rise to a similar profile. Further cycles could not be monitored properly by fluorescence due to sample turbidity, associated with the growth of the baker’s yeast in this medium.
Summarizing, the unprecedented transient formation of soft matter (fibrillary networks) linked to biological activity is achieved. In the described system, baker’s yeast maintains its activity in the presence of the gel network. The success of the system is based on the use of water soluble gelators, whose acidity permits protonation around neutral pH values.
In this dissipative system the fuel, sucrose, is transformed in work, fibrillary network formation, and further dissipated upon CO2 liberation. Therefore, the dissipated energy is associated with an overall entropy gain resulting from the liberation of CO2 to the atmosphere. It is clearly envisaged that the temporal existence of the gels can be regulated by means of sucrose and yeast concentration as well as by the thickness of the polystyrene cap or any other system for the controlled release for CO2. Importantly, this approach is expected to be compatible with other molecular gelators or with the preparation of other soft materials whose formation is linked to pH changes in the range described here. Also, we recall here the important biological relevance of transient fibrillar networks cited in the introduction. Another point of interest is the use of gels with programmed lifetimes for fluidic guidance, release or self-erasing prototypes, as has been smartly pointed out recently.18 Additionally, in a broader scope, life itself is an example of a far-from-equilibrium system, and the development of artificial systems imitating this property is of interest for the study of the origin of life.24
Ministry of Science and Innovation of Spain (grant CTQ2012-37735) and Universitat Jaume I (grant P1.1B2012-25) are thanked for financial support.
Notes and references
- A. Ajayaghosh, V. K. Praveen, C. Vijayakumar and S. J. George, Angew. Chem., Int. Ed., 2007, 46, 6260–6265 CrossRef CAS PubMed.
- C. Vijayakumar, V. K. Praveen and A. Ajayaghosh, Adv. Mater., 2009, 21, 2059–2063 CrossRef CAS.
- V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef CAS PubMed.
- I. Prigogine and G. Nicolis, J. Chem. Phys., 1967, 46, 3542 CrossRef CAS.
- M. McCullagh, M. G. Saunders and G. A. Voth, J. Am. Chem. Soc., 2014, 136, 13053–13058 CrossRef CAS PubMed.
- L. Cassimeris, Curr. Biol., 2009, 19, R174–R176 CrossRef CAS PubMed.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS PubMed.
- S. Banerjee, R. K. Das and U. Maitra, J. Mater. Chem., 2009, 19, 6649–6687 RSC.
- J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed.
- J. A. Hunt, R. Chen, T. Van Veen and N. Bryan, J. Mater. Chem. B, 2014, 2, 5319–5338 RSC.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed.
- J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825–4828 CrossRef CAS PubMed.
- J. Boekhoven, W. E. Hendriksen, G. J. M. Koper, R. Eelkema and J. H. van Esch, Science, 2015, 349, 1075–1079 CrossRef CAS PubMed.
- A. K. Das, I. Maity, H. S. Parmar, T. O. McDonald and M. Konda, Biomacromolecules, 2015, 16, 1157–1168 CrossRef CAS PubMed.
- C. G. Pappas, I. R. Sasselli and R. V. Ulijn, Angew. Chem., Int. Ed., 2015, 54, 8119–8123 CrossRef CAS PubMed.
- A. K. Dambenieks, P. H. Q. Vu and T. M. Fyles, Chem. Sci., 2014, 5, 3396–3403 RSC.
- T. Heuser, E. Weyandt and A. Walther, Angew. Chem., Int. Ed., 2015, 54, 13258–13262 CrossRef CAS PubMed.
- B. N. Meyer, N. R. Ferrigni, J. E. Putnam, L. B. Jacobsen, D. E. Nichols and J. L. McLaughlin, Planta Med., 1982, 45, 31–34 CrossRef CAS PubMed.
- A. Pal, Y. K. Ghosh and S. Bhattacharya, Tetrahedron, 2007, 63, 7334–7348 CrossRef CAS.
- M. Tena-Solsona, B. Escuder, J. F. Miravet, V. Casttelleto, I. W. Hamley and A. Dehsorkhi, Chem. Mater., 2015, 27, 3358–3365 CrossRef CAS.
- R. Csuk and B. I. Glanzer, Chem. Rev., 1991, 91, 49–97 CrossRef CAS.
- Z. Guo, L. J. Lee and D. L. Tomasko, Ind. Eng. Chem. Res., 2008, 47, 9636–9643 CrossRef CAS.
- E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details regarding synthesis, gel formation and characterization. See DOI: 10.1039/c6cc01183g |
| This journal is © The Royal Society of Chemistry 2016 |