Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sensitisation of visible and NIR lanthanide emission by InPZnS quantum dots in bi-luminescent hybrids†
Jennifer K.
Molloy‡
ab,
Christophe
Lincheneau‡
ac,
Maria Moula
Karimdjy‡
ab,
Fabio
Agnese
ac,
Lucia
Mattera
ac,
Christelle
Gateau
ab,
Peter
Reiss
ac,
Daniel
Imbert
*ab and
Marinella
Mazzanti
*d
aUniv. Grenoble Alpes, INAC-SCIB, RICC, F-38000 Grenoble, France
bCEA, INAC-SCIB, RICC, F-38000 Grenoble, France
cCEA, INAC-SPrAM, LEMOH, F-38000 Grenoble, France
dInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland. E-mail: marinella.mazzanti@epfl.ch
First published on 4th March 2016
Abstract
The synthesis of stable hybrid nanoparticles combining InPZnS@ZnSe/ZnS quantum dots (QDs) and grafted lanthanide complexes has been performed using two different approaches in organic and aqueous media. The final bi-luminescent hybrids exhibit LnIII (Ln = Eu and Yb) centred luminescence upon QD excitation, suggesting that an energy transfer occurs from the QD to the lanthanide.
The lanthanide ions have attracted increasing attention for a broad range of applications from material science to bioanalysis due to their remarkable intrinsic photophysical properties (narrow emission lines, large effective Stokes shifts, high resistance to photobleaching).1–3 Intense luminescence and high stability are crucial for the technological applications of lanthanide complexes in the areas of bioanalysis, energy conversion (luminescent dyes or solar concentrators) or in devices such as light emitting diodes.4,5 Low energy excitation is also a requirement for these applications, but due to the low molar absorption coefficient of lanthanide transitions (less than 10 M−1 cm−1) it requires the use of suitable chromophores capable of sensitisation of the LnIII emission (antenna effect). Moreover high quantum yields are obtained when LnIII ions are complexed by well-adapted ligands. Most studies have focused on the sensitisation of lanthanide-based luminescence by directly coordinated ligands through the ligand-based triplet excited state, resulting in highly luminescent EuIII
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–8 and TbIII
6–8 and TbIII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6,9 complexes. d-block and f-block metal complexes are also increasingly used as effective chromophores to sensitise lanthanide luminescence emission both in the visible and near-IR range.10–13
6,9 complexes. d-block and f-block metal complexes are also increasingly used as effective chromophores to sensitise lanthanide luminescence emission both in the visible and near-IR range.10–13
Förster resonance energy transfer (FRET) from visible emitting EuIII and TbIII complexes, as energy donors, to CdSe/ZnS quantum dots (QDs), has been reported and Ln–QD hybrids have proven highly sensitive tools in time-resolved fluoro-immunoassays, and multiplexed diagnostics.14–17 Moreover, bi-luminescent Ln–QD hybrids with energy transfer from the lanthanide to the QD can also find applications as light sources in white LEDs.18
On the other hand, the size-tuneable absorption and emission wavelengths and the large single- and two-photon absorption cross sections make QDs appealing chromophores for the sensitisation of various LnIII ions. However, to date only the luminescence sensitisation of TbIII in doped CdSe and of EuIII in doped InPZnS nanocrystals has been reported.19,20 The sensitisation of LnIII emission by QDs has not been achieved so far in Ln–QD organic/inorganic hybrids. Here, we report the synthesis, physical and chemical characterisation of a new series of bi-luminescent hybrid materials. They are based on InPZnS@ZnSe/ZnS core/shell QDs functionalised with podands or self-assembled lanthanide complexes.
Core/shell InPZnS@ZnSe/ZnS QDs with a hydrodynamic diameter of ≈5.5 nm were synthesised in a two-step procedure building on our earlier work.21 UV-vis absorption, luminescence emission, DLS and TEM analyses (cf. ESI†) are consistent with those reported in the literature.22 The QDs used here showed a PL emission maximum in the wavelength range of 525–540 nm and fluorescence quantum yield (QY) of around 40% in organic solvents (QD-sur, where sur is a mixture of four lipophilic surfactants used during synthesis, see ESI†). After phase transfer to aqueous solution the QY dropped to around 15% (QD-pen, where pen refers to penicillamine coating obtained by replacement of the lipophilic surfactants).
Two different LnIII complexes 1-Ln and 2-Ln were grafted onto the QDs according to the procedure shown in Scheme 1. The complexes 1-Ln (Ln = Eu, Tb, Gd and Yb) were prepared using a multi-step synthesis (ESI,† Scheme S1 and Fig. S1). High thermodynamic stability in water was reported for the closely related [Gd(ebpatcn)] complex and similar stability with respect to ligand dissociation is expected for the complexes 1-Ln.23 The complexes 1-EuIII and 1-TbIII show significant luminescence quantum yields, (10 and 19% in water, 22 and 40% in D2O for EuIII and TbIII, respectively). L2-OctSH and L2-Oct were synthesised from the chelidamic acid following the procedure developed by Di Pietro et al.6 for L2-Oct and a modified procedure for L2-OctSH (Scheme S2, ESI†). Previous work suggested that the close proximity of the Ln complex to the QD surface can facilitate photo-induced electron transfer from the QDs to the complex resulting in the quenching of QD emission.24 Various spacer lengths (3 to 12 carbons)§ were therefore investigated and a chain of eight carbon atoms was found to be optimum. It leads to a high number of grafted complexes while being short enough to enable QD-to-Ln energy transfer processes, and long enough to favour them over electron transfer. The L2-Oct ligands form homoleptic (L2-Oct)3–Ln complexes that are highly stable (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β13 ≈ 19 in methanol).25 Similar results were observed with (L2-OctSH)3–Ln (log
β13 ≈ 19 in methanol).25 Similar results were observed with (L2-OctSH)3–Ln (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β13 ≈ 17 in methanol and log
β13 ≈ 17 in methanol and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β13 ≈ 18.2 in CHCl3) and analogous stability is expected for 2-Ln (Fig. S2, ESI†). The (L2-oct)3–Ln complexes showed high luminescence quantum yields in the case of EuIII (70%) and Tb (96%) and a measurable emission for YbIII.6
β13 ≈ 18.2 in CHCl3) and analogous stability is expected for 2-Ln (Fig. S2, ESI†). The (L2-oct)3–Ln complexes showed high luminescence quantum yields in the case of EuIII (70%) and Tb (96%) and a measurable emission for YbIII.6
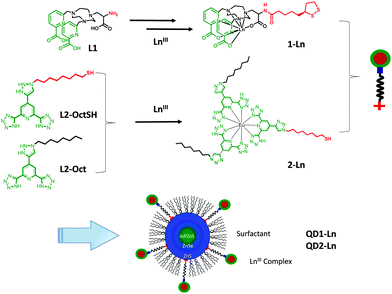 | ||
| Scheme 1 Synthesis of the lanthanide complexes 1-Ln and 2-Ln and of the hybrids QD1-Ln and QD2-Ln (Ln = Eu, Tb, Gd and Yb). | ||
The hybrids QD1-Ln (Ln = Eu, Tb, Yb) were synthesised following a previously reported procedure26via the post functionalisation of QD-pen by the LnIII complexes 1-Ln in the presence of a reducing agent. QD1-Ln (Ln = Eu, Tb, Yb and Gd) were obtained as pure hybrids after size exclusion column chromatography. The number of complexes per QD (115) was determined by combining two techniques, absorption spectroscopy (QD concentration) and magnetic susceptibility (complex concentration). DLS measurements revealed that the QD1-Ln hybrids show a small hydrodynamic diameter of ∼9.5 nm and narrow size distribution with polydispersity indices of an average of 0.4 (Fig. 1 and Fig. S4, Table S1, ESI†). QD-pen exhibits a hydrodynamic diameter, which is 1.5 nm larger than the TEM diameter, corresponding to a 0.75 nm thick ligand shell in accordance with the compact pen-capping. Moreover, the increase of the hydrodynamic diameter of approximately 4 nm from QD-pen to QD1-Ln is consistent with the successful grafting of the complexes at the QD surface. The formation of the hybrids QD2-Ln was performed using a novel three-step strategy relying on the well-defined coordination chemistry of the L2-Oct ligands enabling the controlled supramolecular build-up of the hybrids. It involves purification by extraction and characterisation by absorption and emission spectroscopy at each stage (ESI†). The first step involved a partial exchange in chloroform of the surfactants in QD-sur (TEM, Fig. S5, ESI†) with L2-OctSH to afford the hybrids QD-L2-OctSH (Fig. S6, ESI†). Reaction of QD-L2-OctSH with 1 eq. of Ln(OTf)3 (Ln = Eu, Tb, Yb) per grafted ligand (QD-L2-OctSH·Eu, Fig. S7, ESI†), followed by the addition of 2 eq. of L2-Oct per metal ion afforded the hybrids QD2-Ln (Fig. S8, ESI†). Absorption spectroscopy indicates the presence of 200 grafted complexes per QD.
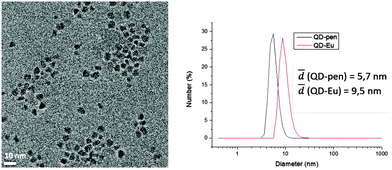 | ||
| Fig. 1 Left: TEM micrographs of QD-pen (mean size: 4.2 ± 0.5 nm). Right: dynamic light scattering data and hydrodynamic diameters of QD-pen and QD1-Eu. | ||
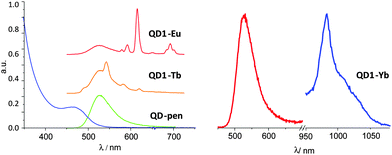
The photophysical properties of QD1-Ln (Fig. 2 and Fig. S9–S11, ESI†) and QD2-Ln (Fig. 3) were then analysed for Ln = Eu, Tb and Yb. After grafting of the ligands or complexes on the surface, the emission QY of the QDs shows a quenching from 40 and 15% for QD-sur and QD-pen to 5–40% and 1–15%, respectively. Direct excitation of QD1-Ln at 273 nm (corresponding to the absorption band of the ligand L1 but also to the absorption of the QD) resulted in the characteristic Eu(5D0 → 7Fj) and Tb(5D4 → 7Fj) luminescence emission of the EuIII and TbIII complexes (Fig. 2, left and ESI†) through ligand excitation. In addition the emission spectra show a band centred at 525 nm resulting from excitation of the QDs.
The excitation at 273 nm, through the absorption band of the ligand, or at 370 nm of the free YbIII complex did not lead to emission in the NIR region. Notably, the presence of a coordinated water molecule leads to the deactivation of the YbIII centred luminescence. However, the free EuIII complex shows a sizeable luminescence upon excitation through the ligand at 273 nm but no emission upon excitation at 370 nm (no residual ligand absorption).
When grafted onto QD-pen, excitation at 273 (excitation of both ligand and QD) and 370 nm (excitation of QD) of QD1·Eu results in a strong and very weak emission of EuIII at 618 nm, respectively. For QD1·Yb, excitation at 370 nm gives a weak luminescence emission despite the presence of the coordinated water molecule. The emission spectrum reveals a band at 978 nm (Fig. 2, right), assigned to the 2F5/2 → 2F7/2 transition and broader vibronic components at longer wavelengths. Excitation at 273 nm of QD1·Yb results in the absence of emission, probably due to a weaker efficiency of both the Xenon lamp (low power) and the gratings (tail) in this region compared to 370 nm. The combination of these two factors makes the YbIII NIR luminescence too weak to be detected at 273 nm as observed for QD1·Eu. The luminescence emission of the lanthanide ions unambiguously arises via excitation of the QDs as no ligand absorption appears at λ > 320 nm. The presence of the QD-sensitised EuIII and YbIII emission was confirmed by the absence of luminescence when the free complexes 1-Ln were excited at 370 nm.
Moreover, when the GdIII ion was replaced by YbIII to form QD1·Yb, the QD lifetimes decreased (3, 16, and 62 ns) compared to the values (4, 23 and 84 ns) measured for QD-pen or QD1-Gd, suggesting the presence of an energy transfer from the QD to the lanthanide ion, associated with a decrease of the QD lifetimes (Table S2, ESI†). For QD1-Eu, the decrease of the QD lifetimes is more pronounced (measured values of 1, 6 and 30 ns) while the EuIII emission lifetime measured in the hybrid QD1-Eu is similar to the one measured for the free complex in water (0.49 and 0.48 ms, respectively). These data indicate that an energy transfer with additional deactivation pathways occurs in QD1-Eu from QD to EuIII. Thus, only a residual emission from the Eu(5D0) level is observed, the emission of the QD and the first accepting Eu(5D0) electronic level being very close in energy (Fig. S9, ESI†). For QD1-Tb (Fig. S10, ESI†), the decrease of the QD lifetimes is significantly less important (3, 19, 72 ns) compared to QD1-Yb and QD1-Eu, so only a small energy transfer might occur, if possible at all. In this case, the emission of the QD overlaps the Tb(5D4) first accepting level with the presence of a back transfer from the Tb(5D4) to the QD (TbIII lifetimes = 0.66 and 0.92 ms for QD1-Tb and 1-Tb, respectively, in aqueous buffer). The nullification of these two energetic pathways could explain the absence of luminescence of TbIII in QD1-Tb. Furthermore, the values of the QDs quantum yields measured for the hybrids QD1-Ln, (1, 5, 10, and 15% for QD1-Eu, -Yb, -Tb and -Gd, respectively, Table S2, ESI†) follow a similar trend to the observed decrease of the lifetimes. This trend matches the decrease of both lifetimes and emission of the donor usually associated to an energy transfer.
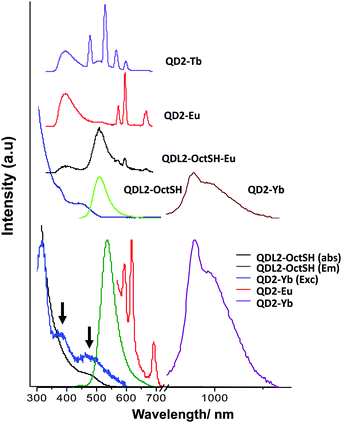
In the case of QD2-L2-OctSH the grafting of the ligands on the surface of the QDs was concomitant with a significant quenching of the QD luminescence emission (decreasing the intensity between 20 and 80% in comparison with a control sample) depending on the quantity of ligand involved in the functionalisation (Fig. 3). The measurements of the QD emission lifetimes (Table S2, ESI†) show a decrease from QD-sur (0.3, 17 and 75 ns) to QD-L2-OctSH (0.2, 5 and 50 ns). The formation of the complex QD-L2-OctSH·Eu leads to a weak luminescence of EuIII, whereas completion of the coordination sphere with L2-Oct affording QD2-Eu leads to luminescence emission similar to that found for the free [Eu(L2-Oct)2(L2-OctSH)] complex and lifetimes for the QD of 0.2, 4 and 31 ns. This suggests that the EuIII coordination environment remains similar to that of the free complex upon grafting at the surface of the QD. The grafting of the 2-Eu and 2-Yb results in large quenching of the QD emission suggesting that a partial energy transfer occurs, while no quenching is observed for 2-Tb (40% QY for QD2-Tb and QD-sur). Excitation of QD2-Tb at 325 nm, (Fig. 3, top), i.e. on both the transitions of the ligand and the QD, results in the emission of both the QD and the lanthanide ions. When excitation of QD2-Tb was performed at 400 nm (where only the QD is excited), the emission of the metal was not observed. This could be due to the Tb(5D4) energy level being too low for an energy transfer or more probably a back-transfer from the Tb(5D4) to the QD level as observed in the case of QD1·Tb. For QD1-Ln and QD2-Ln an energy transfer may not be possible by the resonant transfer mechanism due to a lack of absorption/emission overlap. However, phonon-assisted transfer involving energy mismatch and creation and/or annihilation of phonons could occur.
Conversely, the excitation at low energy (400 nm) of QD2-Eu and QD2-Yb, gave rise to a weak EuIII and a sizeable YbIII luminescence (Fig. 3, bottom). In the YbIII emission spectrum we observe generally one signal in the NIR at approx. 978 nm and a broad component centred at 1040 nm. This is due to an absorption/reemission of the excitation energy by the YbIII energy levels. In addition, the free complexes [Eu(L2-Oct)2(L2-OctSH)] and [Yb(L2-Oct)2(L2-OctSH)] did not show any measurable luminescence emission when excited at 400 nm, indicating that the excitation through the ligand cannot occur at this wavelength. Moreover, the strong luminescence emission of the YbIII in QD2-Yb allowed the measurement of a well-defined excitation spectrum. Thus, the presence of an energy transfer from the QD to the YbIII centre is confirmed by the apparition of two bands at 380 and 450 nm in the excitation spectrum (matching the low-energy band of the absorption spectrum), corresponding to the sensitisation of the complex via the QD. This result confirms that the successful population of the lanthanide energy levels was achieved via sensitisation of the QD core.
Two new types of QD–LnIII organic/inorganic hybrids showing dual luminescence have been prepared and characterised using two different approaches. The QDs and LnIII complexes have been chosen to promote both dual luminescence and energy transfer. A novel self-assembly strategy provides a straightforward and synthetically undemanding route to the synthesis of QD2–Ln hybrids that are soluble and stable in organic solutions. These hybrids are potentially useful for the development of new luminescent materials. The use of functionalised, water-stable lanthanide complexes gave access to QD1-Ln hybrids with potential application in biological imaging. Overall, the obtained QD–LnIII hybrids have allowed the demonstration of the potential of using QDs to sensitise both the visible and NIR emission of lanthanide ions incorporated into QD-grafted complexes, either in organic or aqueous media. Future studies will be directed towards the optimisation of the transfer through an appropriate matching of the Ln and QD energy levels and the thorough investigation of the transfer mechanisms.
The authors gratefully acknowledge financial support from the “French Agence Nationale de la Recherche”, grant NIRA (ANR-13-BS08-0011), Labex Arcane, ANR-11-LABX-003-01 and the Ecole Polytechnique Fédérale de Lausanne (EPFL). They thank Lydia Plassais and Sebastiano Di Pietro for the synthesis of L1 and L2-Oct.
Notes and references
- J.-C. G. Bünzli, Coord. Chem. Rev., 2015, 293, 19–47 CrossRef.
- J.-C. G. Bünzli, Acc. Chem. Res., 2006, 39, 53–61 CrossRef PubMed.
- S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1–31 CrossRef CAS.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- S. Di Pietro, D. Imbert and M. Mazzanti, Chem. Commun., 2014, 50, 10323–10326 RSC.
- G. Nocton, A. Nonat, C. Gateau and M. Mazzanti, Helv. Chim. Acta, 2009, 92, 2257–2273 CrossRef CAS.
- M. Delbianco, V. Sadovnikova, E. Bourrier, G. Mathis, L. Lamarque, J. M. Zwier and D. Parker, Angew. Chem., Int. Ed., 2014, 53, 10718–10722 CrossRef CAS PubMed.
- J. Xu, T. M. Corneillie, E. G. Moore, G.-L. Law, N. G. Butlin and K. N. Raymond, J. Am. Chem. Soc., 2011, 133, 19900–19910 CrossRef CAS PubMed.
- T. J. Sørensen, A. M. Kenwright and S. Faulkner, Chem. Sci., 2015, 6, 2054–2059 RSC.
- S. J. A. Pope, B. J. Coe, S. Faulkner, E. V. Bichenkova, X. Yu and K. T. Douglas, J. Am. Chem. Soc., 2004, 126, 9490–9491 CrossRef CAS PubMed.
- S. Faulkner and S. J. A. Pope, J. Am. Chem. Soc., 2003, 125, 10526–10527 CrossRef CAS PubMed.
- D. Imbert, M. Cantuel, J.-C. G. Bünzli, G. Bernardinelli and C. Piguet, J. Am. Chem. Soc., 2003, 125, 15698–15699 CrossRef CAS PubMed.
- D. Geißler, L. J. Charbonnière, R. F. Ziessel, N. G. Butlin, H.-G. Löehmannsröeben and N. Hildebrandt, Angew. Chem., Int. Ed., 2010, 49, 1396–1401 CrossRef PubMed.
- D. Geißler, S. Linden, K. Liermann, K. D. Wegner, L. J. Charbonnière and N. Hildebrandt, Inorg. Chem., 2014, 53, 1824–1838 CrossRef PubMed.
- N. Hildebrandt, K. D. Wegner and W. R. Algar, Coord. Chem. Rev., 2014, 273, 125–138 CrossRef.
- L. J. Charbonnière and N. Hildebrandt, Eur. J. Inorg. Chem., 2008, 3241–3251 CrossRef.
- B.-H. Kwon, H. S. Jang, H. S. Yoo, S. W. Kim, D. S. Kang, S. Maeng, D. S. Jang, H. Kim and D. Y. Jeon, J. Mater. Chem., 2011, 21, 12812–12818 RSC.
- D. A. Chengelis, A. M. Yingling, P. D. Badger, C. M. Shade and S. Petoud, J. Am. Chem. Soc., 2005, 127, 16752–16753 CrossRef CAS PubMed.
- U. T. D. Thuy, A. Maurice, N. Q. Liem and P. Reiss, Dalton Trans., 2013, 42, 12606–12610 RSC.
- L. Li, M. Protière and P. Reiss, Chem. Mater., 2008, 20, 2621–2623 CrossRef CAS.
- U. T. D. Thuy, P. Reiss and N. Q. Liem, Appl. Phys. Lett., 2010, 97, 193104 CrossRef.
- A. Nonat, M. Giraud, C. Gateau, P. H. Fries, L. Helm and M. Mazzanti, Dalton Trans., 2009, 8033–8046 RSC.
- S. A. Gallagher, S. Comby, M. Wojdyla, T. Gunnlaugsson, J. M. Kelly, Y. K. Gun'ko, I. P. Clark, G. M. Greetham, M. Towrie and S. J. Quinn, Inorg. Chem., 2013, 52, 4133–4135 CrossRef CAS PubMed.
- S. Di Pietro, N. Gauthier, D. Imbert, J. Pécaut and M. Mazzanti, Dalton Trans., 2016, 45, 3429–3442 RSC.
- G. J. Stasiuk, S. Tamang, D. Imbert, C. Poillot, M. Giardiello, C. Tisseyre, E. L. Barbier, P. H. Fries, M. de Waard, P. Reiss and M. Mazzanti, ACS Nano, 2011, 5, 8193–8201 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details for the preparation of ligands and hybrids and spectroscopic characterization. See DOI: 10.1039/c6cc01182a |
| ‡ Contributed equally to this body of work. |
| § Unpublished work: The C3 spacer was not suitable for the formation of the self-assembly due to the sterically encumbered environment around QD-sur. The C12 ligand demonstrates a decrease of the lanthanide sensitisation with respect to the C8 spacer, which was therefore chosen for all further studies. |
| This journal is © The Royal Society of Chemistry 2016 |