Open Access Article
Open Access ArticleAn acido- and photochromic molecular device that mimics triode action†
P.
Remón
a,
S. M.
Li
a,
M.
Grøtli
b,
U.
Pischel
*c and
J.
Andréasson
*a
aDepartment of Chemistry and Chemical Engineering, Physical Chemistry, Chalmers University of Technology, SE-412 96 Gothenburg, Sweden. E-mail: a-son@chalmers.se
bDepartment of Chemistry and Molecular Biology, University of Gothenburg, SE-412 96 Gothenburg, Sweden
cCIQSO - Centre for Research in Sustainable Chemistry and Department of Chemistry, Campus El Carmen, University of Huelva, E-21071 Huelva, Spain. E-mail: uwe.pischel@diq.uhu.es
First published on 7th March 2016
Abstract
The photo-controlled shift of pH titration curves, describing the acidochromic behaviour of a spiropyran switch network, was harnessed for the realisation of a molecular triode. The intricate network can be correctly interpreted with respect to the pH dependence of the main involved species.
The fascinating opportunities offered by light-controlled molecular switches are currently unfolding in research areas such as chemical biology, nanochemistry, materials science, and molecular information processing.1–9 This preference is triggered by the possibility of changing molecular and system's properties by light exposure in a reversible, waste-free, non-invasive, and spatiotemporally controlled fashion. With a view on this, light has been increasingly used as a stimulus to devise molecular devices capable of performing logic operations5,10–14 with different degrees of complexity, e.g., simple logic gates (AND, OR, NOR, etc.), elementary arithmetic operations (addition, subtraction), logic circuits (multiplexer, encoder, etc.), and memory devices.2,15–22
In the semiconductor-driven world triodes/transistors play an archetypal role. Inspired by this, it is very appealing to devise molecules that are capable of mimicking the performance of these analogue electronic building blocks. Despite the conventional use of electrical signalling, chemical and photophysical phenomena can be interpreted along the lines of a molecular triode or transistor as well. One strategy is the exploitation of photochromic (dithienylethene) supramolecular systems for the all-photonic realization of photo-controlled emission modulation via energy transfer.23,24 A different approach was followed using a pH-sensitive fluorophore, whose electron-transfer-induced emission quenching can be additionally controlled by metal cation complexation. The resulting pKa shifts resemble the characteristics of triode i–V curves.25 Finally, very recently the peculiar temperature-dependent operation of a fluorescent electrochemical switch was harnessed for demonstrating a transistor function.26 Some of these systems require either intensive synthetic labour or a very specific chemical design. Others work with metal-cation inputs which may cause problems for the recycling of the function.
In an effort to base our chemical design on the generally and widely used class of photofunctional spiropyrans we came across a so far overlooked photo-controlled hysteresis effect in the pH titration curves describing the integrated acidochromic behaviour of such systems. Their application in bio-relevant contexts such as light-controlled DNA binding27–29 and interactions on proteins,30 bioimaging,31–33 or biosensing34 provides an additional incentive to explore their inherent functional character beyond the photochromic switching itself, for example for devising a molecular triode. This complements the conceptual importance of spiro-pyrans/-oxazines in molecular information processing as exemplified by molecular memories and Fuzzy logics.35–39
Spiropyrans, such as the water-soluble 1, are implied in photo- and acidochromic switching between three states: spiro form (SP), merocyanine (MC), and protonated merocyanine (MCH); Scheme 1.40 In a very acidic solution (pH < 0.5) the protonated spiro form SPH is abundant, while the undesired hydrolysis of the MC form intervenes especially at basic pH (pH > 8).40,41 Therefore we limited our study to an intermediate pH window between 1.5 and 8.
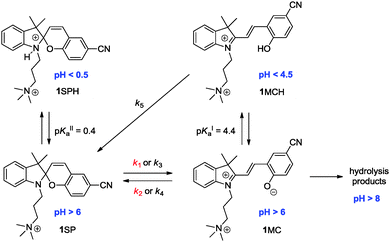 | ||
| Scheme 1 Switch network of spiropyran 1. The associated pH values are approximate and included for the purpose of orientation. The herein relevant rate constants of the thermally-induced processes (red) and photochromic processes (black) are colour-coded; see also ESI†. | ||
In aqueous solution at pH ≥ 6 1SP is in thermal (“dark”) equilibrium with 1MC (K = k1/k2 = 0.16 at pH 7) which can be displaced by protonation of the merocyanine to yield the thermally stable 1MCH (pKaI = 4.4).40 Hence, at high pH (pH ≥ 6) the SP form is dominant, whereas at low pH (pH ≤ 4.5) the MCH form prevails. This is clearly manifested in the corresponding UV/vis absorption spectra (see Fig. 1). The small 1MC absorption band (λmax = 515 nm) at pH 6 is contrasted by the dominating 1MCH absorption band (λmax = 416 nm) at pH 3.
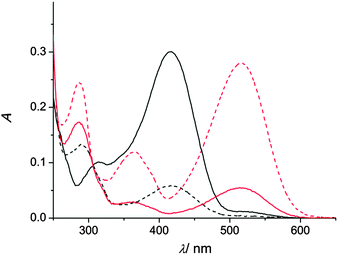 | ||
| Fig. 1 UV/vis absorption spectra of 1 (20 μM in water) for the thermal equilibrium (pH 3, black full line; pH 6, red full line) and in the PSS (pH 3, black dashed line; pH 6, red dashed line). | ||
The situation changes dramatically when UV light (λ = 254 nm, power density of 700 μW cm−2) is applied until reaching the photostationary state (PSS). Now 1SP is photoisomerised to 1MC at pH ≥ 6. The quantum yields (Φ) of the ring-opening and closing paths are 0.040 and 0.012, respectively.‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 However, at pH ≤ 4.5 UV exposure culminates in the enrichment of the sample in 1SP; see Fig. 1. This is reasoned with the highly competitive ring closure of the 1MCH form to 1SP (Φ = 0.21).
40 However, at pH ≤ 4.5 UV exposure culminates in the enrichment of the sample in 1SP; see Fig. 1. This is reasoned with the highly competitive ring closure of the 1MCH form to 1SP (Φ = 0.21).
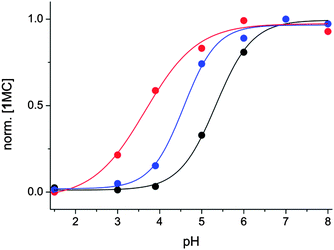
The photo-driven variation of the composition of the switch network has consequences for the titration curve monitoring the normalised concentration of the merocyanine form ([1MC]/[1MC]max) depending on the pH; see Fig. 2. 1MC can be conveniently followed by its absorbance at λ = 515 nm, where none of the other forms of 1 absorb. In the absence of light irradiation, the concentration of 1MC is mainly determined by the degree of protonation producing 1MCH. However, as described above, 1MCH is efficiently photo-converted to 1SP and thereby removed from the protonation equilibrium involving 1MC. Consequently, the re-establishment of the thus disturbed equilibrium leads to a decreased relative 1MC concentration as compared to the same pH in the absence of UV irradiation. This is experimentally expressed in a positive shift of the “apparent pKa” value by about 1.6 units. Note that this shift is the result of the integrated behaviour of the switch network and conceptually different from the observation of pKa shifts on photo-conversion between two isomers with differing basicity.42,43
Furthermore it was foreseen that the irradiation with an attenuated UV intensity would reveal an intermediate situation, signalled by a pH titration curve situated between the two beforehand discussed cases (thermal processes and irradiation at full UV intensity). Gratifyingly this was indeed verified for an attenuation to 1.3% of full UV intensity; see Fig. 2.
The working principle and characteristics of a triode can be described as follows. Upon heating the cathode by a filament a thermoionic emission of electrons results, which is collected by a positively charged anode plate. On their way the electrons have to pass through a grid which acts like a gate. The anode current–voltage (iA–VA) curve can be shifted by the variation of the grid voltage (VG), resulting in the situation shown in Fig. 3. The comparison of these electronic characteristics with the herein observed shift of the pH titration curve allows the drawing of analogies. In our molecular version the triode produces an output in the form of 1MC concentration, which is varied by pH and UV intensity, corresponding to iA, VA, and VG, respectively.
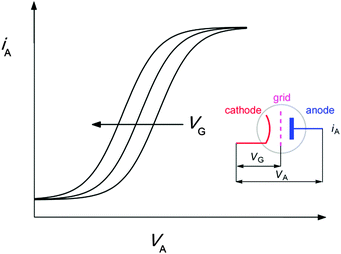 | ||
| Fig. 3 Characteristic i–V curves of a triode upon variation of the grid voltage (VG). The inset shows the electronic representation of a triode and the assignment of the applied potentials. | ||
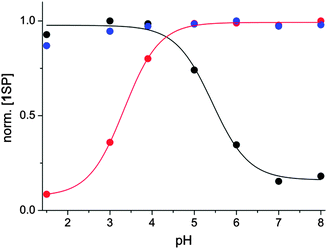
Based on the relevant rate- and protonation constants, the pH titration curve monitoring the concentration of 1MC was modelled and it showed virtually quantitative agreement with the experimental data (see ESI†). The discussed intricate interplay between the different forms of the network was further validated by monitoring the 1SP and 1MCH forms. Their observation is not directly linked to the readout of the absorbance at a specific wavelength as their absorption spectra overlap significantly. Therefore a system of equations that allows the numerical calculation of their concentrations was developed (see ESI†). Expectedly and in accordance with the corresponding modelling, monitoring the normalised concentration of 1MCH revealed opposite pH dependence compared to that established for 1MC, see ESI.† Finally, also the plot of the normalised concentration of 1SP confirmed the applied lines of thought. Interestingly, but not unexpectedly, the titration curves for 1SP are not shifted but inverted (Fig. 4). In the absence of irradiation, the SP concentration is elevated at high pH (the 1SP/1MC equilibrium is on the side of the ring-closed SP form; see above) and diminished at low pH (mostly due to the shifted equilibrium caused by the formation of the thermally stable 1MCH). However, under irradiation this is inverted: the relative concentration of 1SP is high at low pH (due to the efficient 1MCH → 1SP conversion) and low at high pH (due to the 1SP → 1MC conversion). Curiously, the application of attenuated UV intensity balances the network in such a way that virtually no pH dependence of the relative 1SP concentration is observed.
In conclusion, the herein discovered photo-controlled shift of the pH titration curve, describing the acidochromic behaviour of the water-soluble spiropyran 1, can be harnessed for the design of a molecular triode. The output of this device is read through the exclusive absorption signal of the MC form at 515 nm and the grid voltage is presented by the UV intensity. The described effects open the door to integrate the triode function with other features that can be introduced for example on the synthetically flexible indole nitrogen position.
Financial support from the Spanish Ministerio de Economía y Competitividad (grants CTQ2011-28390 and CTQ2014-54729-C2-1-P for U. P.), the Junta de Andalucía (P12-FQM-2140), FEDER, and the Swedish Research Council VR (grant 622-2010-280 for J. A.) is gratefully acknowledged.
Notes and references
- I. Yildiz, S. Impellizzeri, E. Deniz, B. McCaughan, J. F. Callan and F. M. Raymo, J. Am. Chem. Soc., 2011, 133, 871–879 CrossRef CAS PubMed.
- D. Gust, J. Andréasson, U. Pischel, T. A. Moore and A. L. Moore, Chem. Commun., 2012, 48, 1947–1957 RSC.
- M. Natali and S. Giordani, Chem. Soc. Rev., 2012, 41, 4010–4029 RSC.
- R. Tong, H. D. Hemmati, R. Langer and D. S. Kohane, J. Am. Chem. Soc., 2012, 134, 8848–8855 CrossRef CAS PubMed.
- A. P. de Silva, Molecular Logic-based Computation, The Royal Society of Chemistry, Cambridge, 2013 Search PubMed.
- J. J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef CAS PubMed.
- W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- R. Göstl, A. Senf and S. Hecht, Chem. Soc. Rev., 2014, 43, 1982–1996 RSC.
- J. J. Zhang, J. X. Wang and H. Tian, Mater. Horiz., 2014, 1, 169–184 RSC.
- G. de Ruiter and M. E. van der Boom, J. Mater. Chem., 2011, 21, 17575–17581 RSC.
- M. Elstner, K. Weisshart, K. Müllen and A. Schiller, J. Am. Chem. Soc., 2012, 134, 8098–8100 CrossRef CAS PubMed.
- S. Erbas-Cakmak, O. A. Bozdemir, Y. Cakmak and E. U. Akkaya, Chem. Sci., 2013, 4, 858–862 RSC.
- B. Rout, P. Milko, M. A. Iron, L. Motiei and D. Margulies, J. Am. Chem. Soc., 2013, 135, 15330–15333 CrossRef CAS PubMed.
- J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053–1069 RSC.
- S. Silvi, E. C. Constable, C. E. Housecroft, J. E. Beves, E. L. Dunphy, M. Tomasulo, F. M. Raymo and A. Credi, Chem. – Eur. J., 2009, 15, 178–185 CrossRef CAS PubMed.
- J. Kärnbratt, M. Hammarson, S. M. Li, H. L. Anderson, B. Albinsson and J. Andréasson, Angew. Chem., Int. Ed., 2010, 49, 1854–1857 CrossRef PubMed.
- J. Andréasson, U. Pischel, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2011, 133, 11641–11648 CrossRef PubMed.
- P. Remón, M. Bälter, S. M. Li, J. Andréasson and U. Pischel, J. Am. Chem. Soc., 2011, 133, 20742–20745 CrossRef PubMed.
- S. J. Chen, Y. H. Yang, Y. Wu, H. Tian and W. H. Zhu, J. Mater. Chem., 2012, 22, 5486–5494 RSC.
- T. Avellini, H. Li, A. Coskun, G. Barin, A. Trabolsi, A. N. Basuray, S. K. Dey, A. Credi, S. Silvi, J. F. Stoddart and M. Venturi, Angew. Chem., Int. Ed., 2012, 51, 1611–1615 CrossRef CAS PubMed.
- M. Bälter, S. M. Li, J. R. Nilsson, J. Andréasson and U. Pischel, J. Am. Chem. Soc., 2013, 135, 10230–10233 CrossRef PubMed.
- Y. Wu, Y. S. Xie, Q. Zhang, H. Tian, W. H. Zhu and A. D. Q. Li, Angew. Chem., Int. Ed., 2014, 53, 2090–2094 CrossRef CAS PubMed.
- A. E. Keirstead, J. W. Bridgewater, Y. Terazono, G. Kodis, S. Straight, P. A. Liddell, A. L. Moore, T. A. Moore and D. Gust, J. Am. Chem. Soc., 2010, 132, 6588–6595 CrossRef CAS PubMed.
- M. Pärs, C. C. Hofmann, K. Willinger, P. Bauer, M. Thelakkat and J. Köhler, Angew. Chem., Int. Ed., 2011, 50, 11405–11408 CrossRef PubMed.
- A. J. M. Huxley, M. Schroeder, H. Q. N. Gunaratne and A. P. de Silva, Angew. Chem., Int. Ed., 2014, 53, 3622–3625 CrossRef CAS PubMed.
- I. Gallardo, G. Guirado, J. Hernando, S. Morais and G. Prats, Chem. Sci., 2016, 7, 1819–1825 RSC.
- J. Andersson, S. M. Li, P. Lincoln and J. Andréasson, J. Am. Chem. Soc., 2008, 130, 11836–11837 CrossRef CAS PubMed.
- M. Hammarson, J. Andersson, S. M. Li, P. Lincoln and J. Andréasson, Chem. Commun., 2010, 46, 7130–7132 RSC.
- M. Hammarson, J. R. Nilsson, S. M. Li, P. Lincoln and J. Andréasson, Chem. – Eur. J., 2014, 20, 15855–15862 CrossRef CAS PubMed.
- T. Sakata, Y. L. Yan and G. Marriott, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 4759–4764 CrossRef CAS PubMed.
- L. Y. Zhu, W. W. Wu, M.-Q. Zhu, J. J. Han, J. K. Hurst and A. D. Q. Li, J. Am. Chem. Soc., 2007, 129, 3524–3526 CrossRef CAS PubMed.
- G. Marriott, S. Mao, T. Sakata, J. Ran, D. K. Jackson, C. Petchprayoon, T. J. Gomez, E. Warp, O. Tulyathan, H. L. Aaron, E. Y. Isacoff and Y. L. Yan, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17789–17794 CrossRef CAS PubMed.
- Z. Y. Tian, W. W. Wu, W. Wan and A. D. Q. Li, J. Am. Chem. Soc., 2009, 131, 4245–4252 CrossRef CAS PubMed.
- N. Shao, J. Y. Jin, S. M. Cheung, R. H. Yang, W. H. Chan and T. Mo, Angew. Chem., Int. Ed., 2006, 45, 4944–4948 CrossRef CAS PubMed.
- Y. Hirshberg, J. Am. Chem. Soc., 1956, 78, 2304–2312 CrossRef CAS.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741–1754 CrossRef CAS PubMed.
- F. M. Raymo and S. Giordani, J. Am. Chem. Soc., 2001, 123, 4651–4652 CrossRef CAS PubMed.
- F. M. Raymo and S. Giordani, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4941–4944 CrossRef CAS.
- P. L. Gentili, A. L. Rightler, B. M. Heron and C. D. Gabbutt, Chem. Commun., 2016, 52, 1474–1477 RSC.
- M. Hammarson, J. R. Nilsson, S. M. Li, T. Beke-Somfai and J. Andréasson, J. Phys. Chem. B, 2013, 117, 13561–13571 CrossRef CAS PubMed.
- T. Stafforst and D. Hilvert, Chem. Commun., 2009, 287–288 RSC.
- M. V. Peters, R. S. Stoll, A. Kühn and S. Hecht, Angew. Chem., Int. Ed., 2008, 47, 5968–5972 CrossRef CAS PubMed.
- S. Samanta, A. Babalhavaeji, M.-X. Dong and G. A. Woolley, Angew. Chem., Int. Ed., 2013, 52, 14127–14130 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental data, procedures for obtaining the concentrations of the different involved open and closed forms of 1 at each pH for the thermal and photochromic processes, and modelling of the network. See DOI: 10.1039/c6cc00840b |
| ‡ It was assumed that the isomerisation quantum yields are independent of the irradiation wavelength. |
| This journal is © The Royal Society of Chemistry 2016 |