Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Flowerlike WSe2 and WS2 microspheres: one-pot synthesis, formation mechanism and application in heavy metal ion sequestration†
Wei
Li
a,
Dehong
Chen
b,
Fang
Xia
ac,
Jeannie Z. Y.
Tan
ab,
Jingchao
Song
ad,
Wei-Guo
Song
e and
Rachel A.
Caruso
*ab
aCSIRO Manufacturing, The Commonwealth Scientific and Industrial Research Organization (CSIRO), Clayton South, Victoria 3169, Australia
bParticulate Fluids Processing Centre, School of Chemistry, The University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: rcaruso@unimelb.edu.au
cSchool of Engineering and Information Technology, Murdoch University, Murdoch, Western Australia 6150, Australia
dDepartment of Materials Science and Engineering, Monash University, Clayton, Victoria 3800, Australia
eBeijing National Laboratory for Molecular Sciences (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 2nd March 2016
Abstract
Flowerlike WSe2 and WS2 microspheres were synthesized by a facile and scalable one-pot solvothermal method. Their formation mechanism followed the reaction between dissolved W(CO)6 and dissolved S or melted Se without complete decomposition of W(CO)6 into tungsten. As novel efficient sorbents, WSe2 and WS2 demonstrated outstanding uptake capacities for Pb2+ and Hg2+.
Layered transition metal dichalcogenides (TMDs), MX2 where M is a transition metal (such as Mo, W, and Nb) and X is a chalcogen (S, Se and Te), have received intensive research interest due to their unique electronic, optical, mechanical and chemical properties.1 Among them, WSe2 and WS2 have been extensively studied for a variety of applications including valleytronics,2 batteries,3 electrocatalysis,4 photocatalysis,5 bioimaging labels,6 and electronic devices.7 WSe2 and WS2 have a layered structure in the form of Se–W–Se or S–W–S with a W atomic layer sandwiched by two hexagonal chalcogen atomic layers.4a,b The interlayers are coupled by weak van der Waals forces and the intralayer W–Se/S bonding is covalent.1 Most reports focus on the fabrication of nanosheets/nanotubes of WSe2 and WS2 by chemical vapor deposition (CVD), electrochemical exfoliation and hot-injection approaches.2–7 These methods require high temperature, expensive precursors or toxic H2S/H2Se, and harsh conditions that give low yields. The growth mechanisms of TMDs have been rarely described, although the formation mechanisms of similar lamellar bismuth chalcogenide materials have been explored.1e,f Moreover, there are few reports on the construction of three-dimensional (3D) hierarchical nanosheet-assembled flowerlike architectures of WSe2, although 3D flowerlike MoS2 and metal oxides or hydroxides such as FeOOH and AlOOH have been widely studied.8 WS2 nanoflowers were produced by using CVD with low yields.9 Hierarchical nanosheets assembled into flowerlike structures normally show superior performance in batteries and water treatment.8,10 Therefore, it is highly desirable to develop hierarchical WSe2 and WS2 using a facile, cost-effective and scalable approach.
As a new class of heavy metal ion scavenger, metal chalcogenides have been used in heavy metal ion sequestration for water treatment, including ZnS, K2xMnxSn3−xS6, (NH4)4In12Se20, H2xMnxSn3−xS6 and HxNayInSz,11 due to the strong affinity of the chalcogen to heavy metal ions. In this study, flowerlike WSe2 and WS2 microspheres consisting of their corresponding nanosheets were synthesized by a simple, low-cost and high-yield one-pot template-free solvothermal approach. Their growth mechanism was elucidated by in situ synchrotron radiation X-ray diffraction (SR-XRD). WSe2 and WS2 were used in heavy metal ion sequestration for the first time and showed exceptional uptake capacities for Pb2+ and Hg2+, making them ideal candidates for heavy metal remediation in practical water purification.
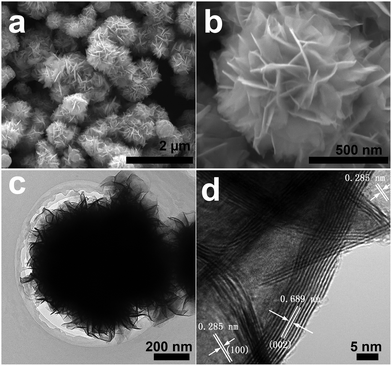
WSe2 and WS2 were prepared in high yields (>3 g) by a solvothermal method from the reaction of W(CO)6 and Se or S powders in p-xylene (see details in ESI†). Fig. 1 shows the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the flowerlike WSe2. The WSe2 sample displays a hierarchical flowerlike micro/nanostructure (Fig. 1). The flowerlike WSe2 is composed of many self-assembled petals that are curly nanosheets (Fig. 1c). These WSe2 nanosheets are 5 nm in thickness and 300 nm in width, and are assembled together to form 3D WSe2 flowers with diameters ranging from 500 nm to 1 μm. Fig. 1d demonstrates that the thin WSe2 nanosheets consist of a few layers (<10 layers) stacking together and the interlayer spacing of the nanosheets is 0.689 nm, corresponding to the (002) plane of the hexagonal WSe2, with slight expansion due to the strain from the layer curvature.3b The lattice spacing of 0.285 nm corresponds to the (100) plane of the 2H–WSe2 phase. The X-ray diffraction (XRD) pattern (Fig. S1a, ESI†) is indexed to the hexagonal 2H–WSe2 structure (JCPDS Card No. 38-1388). The energy dispersive X-ray (EDX) spectroscopy confirms the composition of WSe2 with a W/Se atomic ratio of 0.51 (Fig. S1b, ESI†), in good agreement with the result (W/Se atomic ratio = 0.54) from inductively coupled plasma optical emission spectroscopy (ICP-OES). The X-ray photoelectron spectroscopy (XPS, Fig. S2, ESI†) shows that the W 4f7/2 (31.9 eV) and W 4f5/2 (34.0 eV) peaks are consistent with previous studies of WSe2 samples.4a,12 The small peaks at 35.6 eV and 37.8 eV are assigned to W 4f7/2 and W 4f5/2 from WO3, likely due to oxidation because of exposure to air during sample preparation and transfer for XPS characterization, and were observed in previous WSe2 reports.4a,12 The Se 3d peak was located at 54.4 eV which could be deconvoluted into 3d5/2 and 3d3/2 peaks.
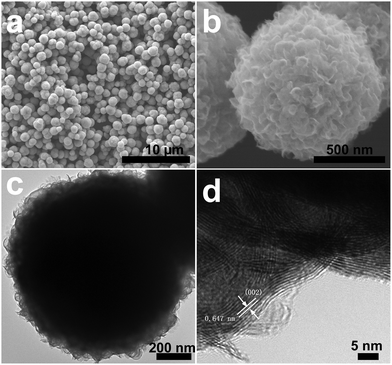
A similar solvothermal approach using sulfur powder instead of selenium produced a WS2 sample. The product is composed of microspheres with an average diameter of 1 μm (Fig. 2a). The WS2 microsphere is comprised of curly nanosheets (Fig. 2b and c), yet with smaller width (several tens of nanometers) compared to that of the WSe2 nanosheets. The WS2 nanosheets consist of 5–10 layers and the interlayer distance is 0.647 nm corresponding to the (002) plane of the hexagonal WS2 (Fig. 2d). The XRD pattern (Fig. S3a, ESI†) can be assigned to the hexagonal 2H–WS2 structure (JCPDS Card No. 84-1398), yet with a small shift in (002) peak towards low angle, which is probably caused by the layer curvature.3b Both the EDX spectroscopy (Fig. S3b, ESI†) and ICP-OES results show the presence of W and S in the WS2 microspheres with a W/S atomic ratio close to 0.5. The XPS spectra (Fig. S4, ESI†) display the W 4f7/2, W 4f5/2 and W 5p5/2 peaks as well as S 2p peak consistent with previous reported WS2 samples, corroborating that the sample is pure WS2 without oxidation.4b,c,5b
These results demonstrate that the solvothermal method is efficient in producing nanosheet self-assembled 3D WSe2 and WS2 micro/nanostructures in high yields. The presence of nanosheets in this architecture enhances the surface-to-volume ratio and offers more accessible interfaces for sequestration, while the entire microstructure enables fast and easy sedimentation and separation from water.8a–c
In order to unravel the growth mechanism of flowerlike WSe2 and WS2 microspheres, the synthesis conditions, including temperature and reaction time, were varied and the samples were characterized by SEM and ex situ XRD.
For WSe2, the optimal temperature is 250 °C, which is above the melting point (m.p.) of Se (221 °C). Below 250 °C, there was residual Se in the product (Fig. S5 & S6, ESI†). WS2 microspheres formed at 150 °C and crystallized upon rising temperature and the morphology evolved from irregular microspheres with smooth surfaces to relatively uniform microspheres with a well-defined nanosheet assembly (Fig. S7, ESI†). At 250 °C, the smooth WSe2 microspheres gradually developed into coarse spheres with improved crystallinity and eventually crystalline flowerlike WSe2 formed (Fig. S8, ESI†), as the reaction time was prolonged. A similar process also occurred in the growth of WS2 microspheres except that the lateral size of the WS2 nanosheets was smaller than that of the WSe2 nanosheets (Fig. S9, ESI†). Therefore, for both WSe2 and WS2, smooth microspheres initially formed and then the nanosheets were developed and crystallized on the surface of microspheres under the solvothermal conditions. Duphil et al. reported an ambient solution method to produce merely amorphous WSe2 and WS2 irregular nanoparticles using W(CO)6, Se and S in p-xylene.13 Pol et al. previously reported a high-temperature solid reaction between W(CO)6 and Se to prepare WSe2 nanoparticles.14 They both proposed a two-step reaction mechanism involving first the complete decomposition of W(CO)6 into W and subsequent reaction of W with Se/S without giving direct evidence. In the absence of Se or S, W(CO)6 was heated at varying temperatures in p-xylene in a Teflon-lined autoclave. The resultant products remained in the phase of W(CO)6 (Fig. S10, ESI†), with no evidence of a W intermediate.
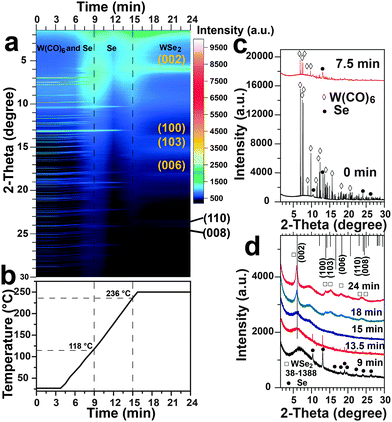
To track the phase evolution as a function of reaction time, time-resolved in situ SR-XRD experiments were performed. The in situ SR-XRD is able to provide valuable real-time phase evolution during the reaction,1e,15 as illustrated in Scheme S1, ESI.† The in situ SR-XRD patterns of WSe2 (Fig. 3) demonstrate that the solvothermal process involves dissolution of W(CO)6, melting of Se and growth of flowerlike WSe2. The in situ SR-XRD patterns (Fig. 2a) of the starting precursors are readily indexed to the orthorhombic phase of W(CO)6 (JCPDS Card No. 40-0752) and hexagonal Se (JCPDS Card No. 06-0362), consistent with ex situ XRD patterns (Fig. S6, ESI†).
Upon heating in p-xylene, the peaks of W(CO)6 continuously decreased in intensity and vanished after about 9 min at 118 °C (Fig. 3b–d). This temperature was much lower than the decomposition/melting point of W(CO)6 (∼170 °C) indicating that W(CO)6 was neither melted nor completely decomposed into elemental tungsten. W(CO)6 was likely dissolved in p-xylene. The peaks of the Se precursor remained until the temperature reached 236 °C (above the melting point of Se, 221 °C), when the Se precursor peak disappeared instantly. WSe2 formed rapidly after the melting of Se. The elemental W intermediate arising from the decomposition of W(CO)6 proposed in the early literature was not detected.13,14 The formation mechanism determined from the in situ SR-XRD result agrees with the aforementioned ex situ SEM and XRD characterization. The heating rate governs the rate of Se melting and hence the whole reaction rate. Therefore, the reaction temperature plays a critical role in the formation of WSe2. Since evidence for the formation of W was not found in either ex situ or in situ experiments, we do not deem that the reaction between dissolved W(CO)6 and melted Se in p-xylene in this case followed the two-step process as proposed in the literature.13,14 The reaction mechanism might be a straightforward one-step reaction, or a modified two-step process initially involving the partial dissociation of W(CO)6 into W(CO)6−x,16 and the subsequent oxidation by Se/S atoms. The underlying mechanism will be investigated in the future through advanced in situ techniques (e.g. mass spectrometry). Besides, agglomerate WSe2 with poor crystallinity (Fig. S11, ESI†) was obtained when W(CO)6 reacted with Se without p-xylene, highlighting the importance of the p-xylene solvent in inducing the formation of flowerlike structures.
The growth process of WS2 was also explored by the in situ SR-XRD (Fig. S12, ESI†). Sulfur (m.p. 115 °C) and W(CO)6 were dissolved at 83 °C and 116 °C, respectively. Then WS2 was gradually formed and crystallized upon heating. Both precursors vanished below their melting or decomposition point and no W intermediate was detected, consistent with the results of WSe2.
The synthesis of WTe2 at 250 °C with the solvothermal method using W(CO)6 and Te (m.p. 450 °C) was attempted. However, the product was only a mixture of Te and TeO2 with W(CO)6, which was removed by rinsing with acetone (Fig. S13, ESI†).
Based on the above ex situ and in situ experimental results, the growth of WS2 and WSe2 began with the dissolution of S and W(CO)6, melting of Se and then both precursors reacted quickly to produce crystalline WS2 and WSe2 under the solvothermal condition. The absence of complete dissociation of W(CO)6 into W in this work appears to defy the commonly accepted two-step reaction mechanism.13,14 The knowledge gained about the growth mechanism of tungsten sulfide and selenide by the time-resolved in situ SR-XRD technique will inspire new studies of various layered transition metal dichalcogenides.
These as-synthesized flowerlike WSe2 and WS2 microspheres possess many nanosheets and abundant chalcogen ligands with innate reactivity towards soft heavy metal ions (Hg2+, Pb2+, etc.) and structural rigidity. These features are desirable for the sequestration of heavy metal ions from water. The flowerlike WSe2 and WS2 microspheres were used to sequester various heavy metal ions, including As(V), As(III), Cd2+, Pb2+ and Hg2+, from water. They did not remove As(V), As(III) and Cd2+, whereas they exhibited high uptake capacities for Hg2+ and Pb2+. This is due to the innate selectivity of metal chalcogenides for soft heavy metal ions (i.e. Hg2+ and Pb2+).11Fig. 4 exhibits the variation in Pb2+ and Hg2+ concentrations before and after sequestration by WSe2 and WS2 samples at 0.5 g L−1. The WSe2 and WS2 were able to reduce the concentration of Pb2+ from 52.30 mg L−1 to 0.76 and 0.42 mg L−1 (Fig. 4a), respectively, giving a Pb2+ uptake capacity of ca. 103 mg g−1. The highest Pb2+ uptake capacities of WSe2 and WS2 are 288 and 386 mg g−1 when treating 1048 mg L−1 of lead ions in water, which are substantially higher than those of nanostructured adsorbents with abundant hydroxyl groups reported previously, such as urchin-like FeOOH (80 mg g−1),8a flowerlike zinc silicate (210 mg g−1) and flowerlike AlOOH (124.2 mg g−1) under similar conditions (Table S1, ESI†).8c,10 Particularly, WSe2 and WS2 show extremely high uptake capacities for Hg2+ (Fig. 4b). WSe2 and WS2 could decrease the concentration of Hg2+ from 978 mg L−1 to 222 mg L−1 and 0.71 mg L−1 resulting in impressive capacities of 1512 mg g−1 and 1954 mg g−1, respectively. Their capacities for Hg2+ are significantly higher than those of conventional metal oxide and carbon-based adsorbents (Table S1, ESI†) and those of metal chalcogenides (Table S2, ESI†).11 Flowerlike WSe2 was characterized after removal of Pb2+ and Hg2+ (Fig. S14 & S15, ESI†). PbWO4 polyhedron nanoparticles are embedded in the WSe2 nanosheets suggesting that Pb2+ ions were removed via a chemical reaction. When treating Hg2+ ions in water, the WSe2 nanosheets were almost fully covered by Hg2Cl2 and Hg3Se2Cl2 nanoparticles, suggesting that WSe2 is a reducing agent that has high reactivity towards Hg2+ leading to the high Hg2+ uptake capacity. Likewise, PbWO4 nanoparticles are immobilized on WS2 nanosheets (Fig. S16 & S17, ESI†). WS2 microspheres are enclosed by as-formed Hg3S2Cl2 and Hg2Cl2 particles, indicating extremely high reactivity and reducibility of WS2 towards Hg2+ ions, which resulted in such a high Hg2+ sequestration capacity. The WS2 microsphere is superior to the flowerlike WSe2 in terms of uptake capacities for Pb2+ and Hg2+, possibly because sulfide has higher reactivity towards Pb2+ and Hg2+ than selenide. This mechanism is different from the direct cation exchange mechanism of other metal chalcogenides for heavy metal ion sequestration.11
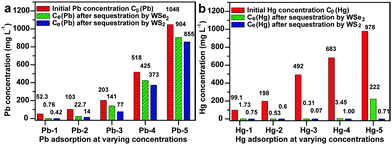 | ||
| Fig. 4 (a) Pb2+ and (b) Hg2+ sequestration of WSe2 and WS2 in water. The WSe2 and WS2 content in the Pb2+ and Hg2+ aqueous solutions was 0.5 g L−1. | ||
In summary, flowerlike WSe2 and WS2 microspheres assembled of nanosheets were synthesized by a facile and high-yield solvothermal method. The in situ SR-XRD gave insights into the growth mechanism of the flowerlike WSe2 and WS2, which is the reaction between dissolved W(CO)6 and dissolved S or melted Se without complete decomposition of W(CO)6 into a W intermediate. Flowerlike WSe2 and WS2 microspheres were used for heavy metal ion sequestration and exhibited remarkable uptake capacities for Pb2+ (288 mg g−1 for WSe2 and 386 mg g−1 for WS2) and Hg2+ (1512 mg g−1 for WSe2 and 1954 mg g−1 for WS2), showing great potential in heavy metal remediation. The synthesis method and SR-XRD characterization in this work offer novel approaches towards rational design and fabrication of hierarchical transition metal dichalcogenides and understanding of their formation mechanism.
This project received financial support from Joint Research Project funding (GJHZ1224) from the Chinese Academy of Sciences and CSIRO. We acknowledge the Australian Synchrotron for the Powder Diffraction beamline access, and CSIRO Minerals for laboratory in situ XRD access. The CSIRO Office of the Chief Executive (OCE) Postdoctoral and Science Leader Schemes are acknowledged for supporting this work. R. A. C. acknowledges the Australian Research Council for a Future Fellowship (FT0990583).
Notes and references
- (a) Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed; (b) X. Huang, Z. Y. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC; (c) R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2015, 48, 56–64 CrossRef CAS PubMed; (d) M. R. Gao, Y. F. Xu, J. Jiang and S. H. Yu, Chem. Soc. Rev., 2013, 42, 2986–3017 RSC; (e) J. Song, F. Xia, M. Zhao, Y. L. Zhong, W. Li, K. P. Loh, R. A. Caruso and Q. Bao, Chem. Mater., 2015, 27, 3471–3482 CrossRef CAS; (f) X. Liu, J. Xu, Z. Fang, L. Lin, Y. Qian, Y. Wang, C. Ye, C. Ma and J. Zeng, Nano Res., 2015, 8, 3612–3620 CrossRef CAS.
- J. Kim, X. P. Hong, C. H. Jin, S. F. Shi, C. Y. S. Chang, M. H. Chiu, L. J. Li and F. Wang, Science, 2014, 346, 1205–1208 CrossRef CAS PubMed.
- (a) B. Liu, T. Luo, G. Y. Mu, X. F. Wang, D. Chen and G. Z. Shen, ACS Nano, 2013, 7, 8051–8058 CrossRef CAS PubMed; (b) D. Chen, G. Ji, B. Ding, Y. Ma, B. Qu, W. Chen and J. Y. Lee, Nanoscale, 2013, 5, 7890–7896 RSC.
- (a) H. T. Wang, D. S. Kong, P. Johanes, J. J. Cha, G. Y. Zheng, K. Yan, N. A. Liu and Y. Cui, Nano Lett., 2013, 13, 3426–3433 CrossRef CAS PubMed; (b) K. Xu, F. M. Wang, Z. X. Wang, X. Y. Zhan, Q. S. Wang, Z. Z. Cheng, M. Safdar and J. He, ACS Nano, 2014, 8, 8468–8476 CrossRef CAS PubMed; (c) L. Cheng, W. J. Huang, Q. F. Gong, C. H. Liu, Z. Liu, Y. G. Li and H. J. Dai, Angew. Chem., Int. Ed., 2014, 53, 7860–7863 CrossRef CAS PubMed.
- (a) J. R. McKone, A. P. Pieterick, H. B. Gray and N. S. Lewis, J. Am. Chem. Soc., 2013, 135, 223–231 CrossRef CAS PubMed; (b) Y. H. Sang, Z. H. Zhao, M. W. Zhao, P. Hao, Y. H. Leng and H. Liu, Adv. Mater., 2015, 27, 363–369 CrossRef CAS PubMed.
- L. X. Lin, Y. X. Xu, S. W. Zhang, I. M. Ross, A. C. M. Ong and D. A. Allwood, ACS Nano, 2013, 7, 8214–8223 CrossRef CAS PubMed.
- (a) T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y. J. Kim, A. Gholinia, S. J. Haigh, O. Makarovsky, L. Eaves, L. A. Ponomarenko, A. K. Geim, K. S. Novoselov and A. Mishchenko, Nat. Nanotechnol., 2013, 8, 100–103 CrossRef CAS PubMed; (b) H. Fang, S. Chuang, T. C. Chang, K. Takei, T. Takahashi and A. Javey, Nano Lett., 2012, 12, 3788–3792 CrossRef CAS PubMed.
- (a) B. Wang, H. Wu, L. Yu, R. Xu, T.-T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed; (b) H. Li, W. Li, Y. Zhang, T. Wang, B. Wang, W. Xu, L. Jiang, W. Song, C. Shu and C. Wang, J. Mater. Chem., 2011, 21, 7878–7881 RSC; (c) Y.-X. Zhang, Y. Jia, Z. Jin, X.-Y. Yu, W.-H. Xu, T. Luo, B.-J. Zhu, J.-H. Liu and X.-J. Huang, CrystEngComm, 2012, 14, 3005–3007 RSC; (d) S. Hu, W. Chen, J. Zhou, F. Yin, E. Uchaker, Q. Zhang and G. Cao, J. Mater. Chem. A, 2014, 2, 7862–7872 RSC; (e) W. Li, D. H. Chen, F. Xia, J. Z. Y. Tan, P.-P. Huang, W.-G. Song, N. M. Nursam and R. A. Caruso, Environ. Sci.: Nano, 2016, 3, 94–106 RSC; (f) X. Li, W. Li, M. Li, P. Cui, D. H. Chen, T. Gengenbach, L. Chu, H. Liu and G. Song, J. Mater. Chem. A, 2015, 3, 2762–2769 RSC; (g) C. Chen, Y. Yu, W. Li, C. Cao, P. Li, Z. Dou and W.-G. Song, J. Mater. Chem., 2011, 21, 12836–12841 RSC.
- (a) A. Prabakaran, F. Dillon, J. Melbourne, L. Jones, R. J. Nicholls, P. Holdway, J. Britton, A. A. Koos, A. Crossley, P. D. Nellist and N. Grobert, Chem. Commun., 2014, 50, 12360–12362 RSC; (b) Z. L. Yang, D. Q. Gao, J. Zhang, Q. Xu, S. P. Shi, K. Tao and D. S. Xue, Nanoscale, 2015, 7, 650–658 RSC.
- J. Qu, C.-Y. Cao, Y.-L. Hong, C.-Q. Chen, P.-P. Zhu, W.-G. Song and Z.-Y. Wu, J. Mater. Chem., 2012, 22, 3562–3567 RSC.
- (a) I. R. Pala and S. L. Brock, ACS Appl. Mater. Interfaces, 2012, 4, 2160–2167 CrossRef CAS PubMed; (b) M. J. Manos, C. D. Malliakas and M. G. Kanatzidis, Chem. – Eur. J., 2007, 13, 51–58 CrossRef CAS PubMed; (c) M. J. Manos, V. G. Petkov and M. G. Kanatzidis, Adv. Funct. Mater., 2009, 19, 1087–1092 CrossRef CAS; (d) C. W. Abney, J. C. Gilhula, K. Lu and W. Lin, Adv. Mater., 2014, 26, 7993–7997 CrossRef CAS PubMed; (e) M. J. Manos and M. G. Kanatzidis, Chem. – Eur. J., 2009, 15, 4779–4784 CrossRef CAS PubMed.
- N. D. Boscher, C. J. Carmalt and I. P. Parkin, J. Mater. Chem., 2006, 16, 122–127 RSC.
- D. Duphil, S. Bastide, J. C. Rouchaud, J. L. Pastol, B. Legendrel and C. Levy-Clement, Nanotechnology, 2004, 15, 828–832 CrossRef CAS.
- S. V. Pol, V. G. Pol, J. M. Calderon-Moreno and A. Gedanken, J. Phys. Chem. C, 2008, 112, 5356–5360 CAS.
- W. Li, F. Xia, J. Qu, P. Li, D. H. Chen, Z. Chen, Y. Yu, Y. Lu, R. A. Caruso and W.-G. Song, Nano Res., 2014, 7, 903–916 CrossRef CAS.
- N. Wiberg and A. F. Holleman, Inorganic Chemistry, 2001, Academic Press Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc00577b |
| This journal is © The Royal Society of Chemistry 2016 |