Open Access Article
Open Access ArticleX-ray absorption study of ceria nanorods promoting the disproportionation of hydrogen peroxide†
Tai-Sing
Wu‡
ab,
Yunyun
Zhou‡
c,
Renat F.
Sabirianov
d,
Wai-Ning
Mei
d,
Yun-Liang
Soo
*ab and
Chin Li
Cheung
*c
aDepartment of Physics, National Tsing-Hua University, Hsinchu, 30013, Taiwan. E-mail: soo@phys.nthu.edu.tw
bNational Synchrotron Radiation Research Center, Hsinchu, 30013, Taiwan
cDepartment of Chemistry, University of Nebraska-Lincoln, Lincoln, NE 68588, USA. E-mail: ccheung2@unl.edu
dDepartment of Physics, University of Nebraska-Omaha, Omaha, NE 68182, USA
First published on 7th March 2016
Abstract
A quasi in situ X-ray absorption study demonstrated that the disproportionation of hydrogen peroxide (H2O2) promoted by ceria nanorods was associated with a reversible Ce3+/Ce4+ reaction and structural transformations in ceria. The direction of this reversible reaction was postulated to depend on the H2O2 concentration and the fraction of Ce3+ species in ceria nanorods.
Fluorite-structured cerium oxide (or ceria) nanomaterials have attracted great interest from chemistry, materials and engineering communities because of their broad applications in catalysis,1–3 sensors,4 biomedicine,5 and fuel cells.6 Ceria has long been known to generate reactive oxygen species by catalyzing the disproportionation of hydrogen peroxide (H2O2). This system has displayed enhanced catalytic activities towards the degradation of pollutants.7,8 Ceria nanomaterials have also been reported to scavenge free oxygen radicals and reduce toxic H2O2 molecules in biological systems.9 These chemical properties of ceria nanomaterials have been ascribed to the interconversion of the cerium ions between their +3 and +4 states coupled with the formation of oxygen vacancy defects in ceria.10,11 However, this proposed reaction mechanism is still under intense debates.7–9,12
The ability to monitor the chemical state transformation of cerium in ceria during reactions is critical to understand the structure–activity relationships of ceria nanomaterials. Currently, the determination of chemical state transformation of cerium in ceria in aqueous reactions is still challenging, largely because of the interference of water molecules in the measurements. X-ray photoelectron spectroscopy (XPS) and UV-Vis spectroscopy have been applied to evaluate changes in the oxidation states of cerium in ceria after its reaction with aqueous H2O2.12,13 However, due to the altered experimental environments such as the vacuum requirement in XPS and limited quantitative capability of UV/Vis spectroscopy, artefacts and misinterpretations in studies using these techniques are often hard to identify.
In situ X-ray absorption spectroscopy (XAS) has been regarded as a versatile tool to elucidate changes in atomic structures and oxidation states of catalysts during reactions.14 For example, Wang et al. utilized this technique to illustrate metallic copper in the Cu/ceria catalyst as the active species in catalyzing the water gas shift reaction.15 Quasi in situ XAS techniques have also been applied in catalysis studies to overcome challenges such as chemical compatibility of reactors and strong photon absorption by reactors in the implementations of in situ XAS techniques.14,16 For instance, Bergmann et al. applied the quasi in situ XAS technique to reveal reversible structural changes of a crystalline Co3O4 catalyst in an oxygen evolution reaction.16
Herein we report our quasi in situ XAS study of the oxidation states of cerium and the local structures in ceria nanorods upon catalyzing the disproportionation of H2O2. Ceria nanorods were selected over ceria nanoparticles in this study because they are often reported to have higher catalytic activities.1,17 In our XAS experiments, a wet chemical environment enabled by a tris(hydroxymethyl)aminomethane (Tris) buffer solution was employed to prevent the reaction system from drying out and to maintain the pH of the system. Since the reaction mechanism strongly depends upon the pH of the reaction,7,18 this method allows reliable quantitative evaluation of reaction species in ceria samples.11,19,20 Our study revealed reversible changes in the oxidation states of cerium and local atomic structures of ceria in the H2O2 disproportionation reaction. In our experiments, ceria nanorods were first added to a solution containing 10 mM H2O2 and 0.1 M Tris buffer (pH = 7.54) to yield a reaction mixture with a nanorod concentration of 500 mg L−1. Samples of this suspended mixture were pipetted out at various reaction times (T = 10 min to 10 h) to wet filtered papers which were then analyzed by XAS. A control experiment without H2O2 was used for comparisons. These control data were regarded as our data at T = 0 h (see experimental details in the ESI†).
The initial morphology, microstructure and chemical state of as-synthesized ceria nanorods1,3 were characterized by transmission electron microscopy, X-ray diffraction (XRD) and XPS (see Fig. S1, ESI†). The ceria nanorods were 50–200 nm in length and 5–12 nm in diameter. The XRD pattern of the nanorods was indexed according to the ICDD card 04-013-4361 and was found to display a cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure of CeO2. The oxidation states of surface cerium atoms were qualitatively analyzed by XPS. The fraction of Ce3+ on the surface was determined to be ca. 20%, indicating a defective surface structure of this as-synthesized ceria sample (Fig. S3, ESI†).
m structure of CeO2. The oxidation states of surface cerium atoms were qualitatively analyzed by XPS. The fraction of Ce3+ on the surface was determined to be ca. 20%, indicating a defective surface structure of this as-synthesized ceria sample (Fig. S3, ESI†).
X-ray absorption near edge structure (XANES) experiments were performed to investigate the redox behavior of ceria nanorods catalyzing the H2O2 disproportionation reaction. The Ce L3-edge XANES spectra of ceria nanorods were collected at different reaction times during a 10 h reaction. Four of the XANES data scanned at the reaction times of 0 h, 0.5 h, 2 h and 10 h are illustrated in Fig. 1. The differences in the intensities of these spectra were demonstrated by the zoomed-in areas of the peaks. The intensity of the XANES spectrum of ceria nanorods significantly decreased in the first 0.5 h. After another 1.5 h of reaction, the intensity returned to its original magnitude. This was indicated by the XANES data collected at T = 2 h, which had a similar intensity to the one at T = 0 h. As the reaction further proceeded, the XANES spectrum at T = 10 h demonstrated negligible changes in both the spectral intensity and shape when compared with those at T = 0 h and T = 2 h.
 | ||
| Fig. 1 Ce L3-edge XANES spectra of ceria nanorods reacted with 10 mM H2O2 at different reaction times: T = 0 h, 0.5 h, 2 h and 10 h. (inset) Zoomed-in peak areas. | ||
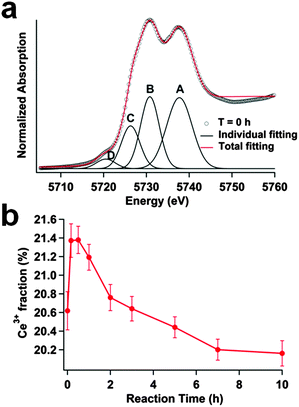
To reveal the changes in the Ce oxidation state, a quantitative analysis was performed by curve-fitting the XANES spectra with an arctangent function to simulate the edge jump and Gaussian functions for peak features21–23 (Fig. 2a). The center of the arctangent function was set at the inflection point of the main edge. Peaks A and B at 5737.7 eV and 5730.8 eV were associated with the Ce4+ ions in ceria, corresponding to the 2p4f05d* and 2p4f15d*L final states, respectively. While 2p denotes the hole produced in 2p3/2, 5d* refers to the presence of an excited electron in the 5d band, and L represents a ligand hole in the anion orbital. Peak C, at 5726.3 eV, was associated with the Ce3+ valence state. Peak D, located at 5720.5 eV in the pre-edge region, was assigned to the final states of 2p4f*, which was forbidden by the selection rule due to a 5d ad-mixture with the 4f state.19,24,25 With a delocalized d character at the bottom of the conduction band due to the cubic crystal-field splitting of Ce 4f states, the transition was partially allowed to appear as a small peak. 4f* referred to the presence of an excited electron in the 4f band.
The Ce L3-edge XANES spectra of all samples showed the coexistence of Ce3+ and Ce4+ states, with the Ce4+ state being dominant in all samples. The fractions of Ce3+ and Ce4+ in the samples were calculated using the following equations:
| [Ce3+] = A(Ce3+)/(A(Ce3+) + A(Ce4+)) |
| [Ce4+] = A(Ce4+)/(A(Ce3+) + A(Ce4+)) |
In summary, during the 10 h long H2O2 disproportionation reaction, the surface Ce4+ of ceria nanorods was reduced to Ce3+ by the H2O2 molecules in the beginning of the first 0.5 h, and then was slowly oxidized back to Ce4+ in the next 9 h. The surface reaction in aqueous solutions was governed by the surface potential and the overall redox potential of ceria and H2O2.9 High concentration of H2O2 exhibited high reduction potential and thus was expected to reduce surface Ce4+ to Ce3+ at the beginning. The slow oxidative process of ceria was likely due to the oxidizing power of H2O2, which became dominant after a dramatic decrease of H2O2 concentration in the solution. In addition, the defective ceria surface with high concentration of Ce3+ decreased the reduction potential of the ceria surface, and was postulated to enable ceria to be oxidized by H2O2.
Local structures surrounding Ce atoms were probed using the extended X-ray absorption fine structure (EXAFS) technique. The experimental data were analyzed using the IFEFFIT software package.26 The Fourier transforms of Ce L3-edge k3-weighted χ(k) of EXAFS spectra depicted the changes in the local structure of Ce atoms with respect to the reaction time (Fig. 3 and Fig. S4, ESI†). The fitting parameters of EXAFS spectra are shown in Table 1 and Table S1 (ESI†). The as-synthesized ceria nanorods exhibited a coordination number of 5.8 in the first Ce–O shell. An extra peak appeared at around 1.65 Å after the addition of hydrogen peroxide. Owing to the “shortness” of this R value, this peak could be attributed to the presence of superficial chemical structures with a Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, possibly similar to the one reported in a cerium(IV) oxo complex, [Ce
O bond, possibly similar to the one reported in a cerium(IV) oxo complex, [Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(LOEt)2(H2O)]·MeC(O)NH2.27 This peak attained its highest intensity at T = 0.5 h. The intensity then slowly decreased after 2 h reaction time until T = 10 h. This trend was consistent with the changes in Ce3+ fraction from XANES data analysis, indicating the modification of local structures caused by reactions with H2O2. The coordination number of the Ce–O bond at 2.34 Å reached its minimum value of 4.7 at T = 0.5 and 2 h, and slowly increased to 5.8 at T = 10 h, which was closer to that of ceria samples before reaction (i.e. at T = 0 h).
O(LOEt)2(H2O)]·MeC(O)NH2.27 This peak attained its highest intensity at T = 0.5 h. The intensity then slowly decreased after 2 h reaction time until T = 10 h. This trend was consistent with the changes in Ce3+ fraction from XANES data analysis, indicating the modification of local structures caused by reactions with H2O2. The coordination number of the Ce–O bond at 2.34 Å reached its minimum value of 4.7 at T = 0.5 and 2 h, and slowly increased to 5.8 at T = 10 h, which was closer to that of ceria samples before reaction (i.e. at T = 0 h).
| Ceria nanorods reaction time | Atom | N | R (Å) | σ 2 (10−3 Å2) |
|---|---|---|---|---|
| T = 0 h | O | 5.8 ± 0.6 | 2.30 ± 0.01 | 4.7 ± 1.2 |
| Ce | 5.6 ± 0.4 | 3.83 | 1.8 ± 0.6 | |
| T = 0.5 h | O | 0.9 ± 0.3 | 1.65 ± 0.02 | 3.9 ± 0.8 |
| O | 4.7 ± 0.4 | 2.32 ± 0.01 | 3.9 ± 0.8 | |
| Ce | 4.7 ± 0.4 | 3.83 | 1.3 ± 0.3 | |
| T = 2 h | O | 0.7 ± 0.2 | 1.66 ± 0.02 | 3.6 ± 0.8 |
| O | 4.7 ± 0.3 | 2.33 ± 0.01 | 3.6 ± 0.8 | |
| Ce | 4.5 ± 0.3 | 3.83 | 0.6 ± 0.4 | |
| T = 10 h | O | 0.7 ± 0.3 | 1.68 ± 0.03 | 6.6 ± 0.8 |
| O | 5.8 ± 0.4 | 2.33 ± 0.01 | 6.6 ± 0.8 | |
| Ce | 4.9 ± 0.3 | 3.83 | 1.1 ± 0.4 | |
The redox cycle of the ceria sample revealed by the EXAFS data agreed well with the intensity changes in the XANES data, implying a reduction-and-oxidation cycle of ceria with H2O2 during the 10 h reaction. Based on our XANES and EXAFS results, we proposed that ceria underwent a redox cycle process in the Fenton-like reaction with H2O2 as follows:
Ceria reduction process:
| Ce4+ + H2O2 → Ce3+ + HO2˙ + H+ | (1) |
| 2Ce3+ + H2O2 + 2H+ → 2Ce4+ + 2H2O | (2) |
| 4Ce3+ + O2 + 4H+ → 4Ce4+ + 2H2O | (3) |
The hydroperoxyl radical (HO2˙) byproduct in eqn (1) was known to involve in the conversion of the Ce oxidation state in ceria.7,28 Besides the reaction species described in eqn (1), other reactive oxygen species such as O2˙, 1O2, O2− and OH˙ had been proposed to form in the reduction reaction.7,9,11 These unstable oxygen species could undergo interconversions with each other through electron transfers. Overall, these species altered the surface coordination and chemical potential of ceria, and therefore regulated the chemical states of cerium in ceria nanorods during the reaction. A slight reduction of its pH value from 7.54 to 7.41 in this system was observed after the first 10 min of the reaction. This finding supported our hypothesis that Ce4+ in ceria were reduced to Ce3+ by H2O2 and H3O+ were produced in this reaction. Our finding corroborated similar observations of pH reduction by Wang et al. in their study of H2O2 disproportionation catalyzed by nanoceria.11
As the reaction proceeded, the concentration of H2O2 decreased and ceria nanorods possessed significant population of Ce3+ on their surfaces. These two factors led to changes in solution potential and ceria surface potential, which consequently promoted the oxidation of Ce3+ to Ce4+ by H2O2 molecules (eqn (2)). During the H2O2 decomposition process, a lot of bubbles which were ascribed to oxygen evolution were observed. This increased the partial pressure of oxygen which could also oxidize the ceria surface (eqn (3)). Overall, these factors possibly gave rise to the decrease in the Ce3+ fraction and an increase in the Ce–O coordination number at 2.3 Å as indicated by our EXAFS data. Furthermore, the coordinatively unsaturated Ce sites, which were indirectly inferred from the increase in Ce3+ fraction, might also provide reactive sites for the adsorption of peroxide species and participate in the disproportionation of H2O2 molecules.11
Our hypothesized redox cycle could also be conjectured from the reported Pourbaix diagram of the Ce(III/IV)–H2O–H2O2 aqueous system.29 Depending on the pH of solutions, H2O2 concentration and the surface potential of ceria, H2O2 could exhibit dual behavior, either acting as an oxidizing agent or a reducing agent.29 The standard potentials of H2O2/HO2˙ and Ce4+/Ce3+ are 1.5 V and 1.44 V, respectively.28 The similarity of these two electrochemical potentials potentially permitted the reversible reaction (eqn (1)–(3)) to occur because the concentration of H2O2 and the Ce3+ fraction of the nanorod surface changed during the catalyzed reaction.
To summarize, we demonstrated the application of a quasi in situ XAS technique to elucidate the changes in chemical states of cerium and local structures in ceria nanorods in their reaction with H2O2. Our measurements under wet conditions allowed reliable analysis of chemical states of cerium and the structures in ceria due to minimal environmental modifications and disturbance to the ceria nanorods. The observed reversible structural changes of ceria nanorods were strongly correlated with the Ce3+/Ce4+ conversion and changes in the H2O2 concentration.
The authors are grateful for the financial support from the National Science Foundation (CHE-1362916). We acknowledge the National Synchrotron Radiation Research Center, Nebraska Center of Materials and Nanoscience, Cornell Center for Materials Research and Rare Earth Salts for the use of their facilities.
Notes and references
- N. J. Lawrence, J. R. Brewer, L. Wang, T. S. Wu, J. Wells-Kingsbury, M. M. Ihrig, G. H. Wang, Y. L. Soo, W. N. Mei and C. L. Cheung, Nano Lett., 2011, 11, 2666–2671 CrossRef CAS PubMed.
- Y. Zhou, N. J. Lawrence, L. Wang, L. M. Kong, T. S. Wu, J. Liu, Y. Gao, J. R. Brewer, V. K. Lawrence, R. F. Sabirianov, Y. L. Soo, X. C. Zeng, P. A. Dowben, W. N. Mei and C. L. Cheung, Angew. Chem., Int. Ed., 2013, 52, 6936–6939 CrossRef CAS PubMed.
- Y. Zhou, N. J. Lawrence, T. S. Wu, J. Liu, P. Kent, Y. L. Soo and C. L. Cheung, ChemCatChem, 2014, 6, 2937–2946 CrossRef CAS.
- N. Izu, S. Nishizaki, W. Shin, T. Itoh, M. Nishibori and I. Matsubara, Sensors, 2009, 9, 8884–8895 CrossRef CAS PubMed.
- C. Xu and X. G. Qu, NPG Asia Mater., 2014, 6, e90 CrossRef CAS.
- S. D. Park, J. M. Vohs and R. J. Gorte, Nature, 2000, 404, 265–267 CrossRef CAS PubMed.
- P. F. Ji, L. Z. Wang, F. Chen and J. L. Zhang, ChemCatChem, 2010, 2, 1552–1554 CrossRef CAS.
- Y. C. Wang, X. X. Shen and F. Chen, J. Mol. Catal. A: Chem., 2014, 381, 38–45 CrossRef CAS.
- S. M. Hirst, A. S. Karakoti, R. D. Tyler, N. Sriranganathan, S. Seal and C. M. Reilly, Small, 2009, 5, 2848–2856 CrossRef CAS PubMed.
- C. Walkey, S. Das, S. Seal, J. Erlichman, K. Heckman, L. Ghibelli, E. Traversa, J. F. McGinnis and W. T. Self, Environ. Sci.: Nano, 2015, 2, 33–53 RSC.
- Y. J. Wang, H. Dong, G. M. Lyu, H. Y. Zhang, J. Ke, L. Q. Kang, J. L. Teng, L. D. Sun, R. Si, J. Zhang, Y. J. Liu, Y. W. Zhang, Y. H. Huang and C. H. Yan, Nanoscale, 2015, 7, 13981–13990 RSC.
- J. M. Perez, A. Asati, S. Nath and C. Kaittanis, Small, 2008, 4, 552–556 CrossRef CAS PubMed.
- M. Das, S. Patil, N. Bhargava, J. F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS PubMed.
- A. I. Frenkel, J. A. Rodriguez and J. G. G. Chen, ACS Catal., 2012, 2, 2269–2280 CrossRef CAS.
- X. Wang, J. A. Rodriguez, J. C. Hanson, D. Gamarra, A. Martínez-Arias and M. Fernández-García, J. Phys. Chem. B, 2006, 110, 428–434 CrossRef CAS PubMed.
- A. Bergmann, E. Martinez-Moreno, D. Teschner, P. Chernev, M. Gliech, J. F. de Araujo, T. Reier, H. Dau and P. Strasser, Nat. Commun., 2015, 6, 8625 CrossRef CAS PubMed.
- X. Du, D. Zhang, L. Shi, R. Gao and J. Zhang, J. Phys. Chem. C, 2012, 116, 10009–10016 CAS.
- P. Salgado, V. Melin, D. Contreras, Y. Moreno and H. D. Mansilla, J. Chil. Chem. Soc., 2013, 58, 2096–2101 CrossRef CAS.
- A. M. Shahin, F. Grandjean, G. J. Long and T. P. Schuman, Chem. Mater., 2005, 17, 315–321 CrossRef CAS.
- F. Zhang, P. Wang, J. Koberstein, S. Khalid and S. W. Chan, Surf. Sci., 2004, 563, 74–82 CrossRef CAS.
- R. C. Karnatak, J. M. Esteva and H. Dexpert, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 1745–1749 CrossRef CAS.
- H. Dexpert, R. C. Karnatak, J. M. Esteva, J. P. Connerade, M. Gasgnier, P. E. Caro and L. Albert, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 1750–1753 CrossRef CAS.
- A. Bianconi, A. Marcelli, H. Dexpert, R. Karnatak, A. Kotani, T. Jo and J. Petiau, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 806–812 CrossRef CAS.
- F. F. Munoz, L. M. Acuna, C. A. Albornoz, A. G. Leyva, R. T. Baker and R. O. Fuentes, Nanoscale, 2015, 7, 271–281 RSC.
- G. Kaindl, G. Schmiester, E. V. Sampathkumaran and P. Wachter, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 10174–10177 CrossRef CAS.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 322–324 CrossRef CAS PubMed.
- Y. M. So, G. C. Wang, Y. Li, H. H. Y. Sung, I. D. Williams, Z. Y. Lin and W. H. Leung, Angew. Chem., Int. Ed., 2014, 53, 1626–1629 CrossRef CAS PubMed.
- P. B. Sigler and B. J. Masters, J. Am. Chem. Soc., 1957, 79, 6353–6357 CrossRef CAS.
- P. Yu, S. A. Hayes, T. J. O'Keefe, M. J. O'Keefe and J. O. Stoffer, J. Electrochem. Soc., 2006, 153, C74–C79 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, microstructure and electronic property analysis by TEM, XRD, XPS and XAS. See DOI: 10.1039/c5cc10643e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |