Open Access Article
Open Access ArticlePhotoactivatable CO release from engineered protein crystals to modulate NF-κB activation†
Hiroyasu
Tabe
a,
Takuya
Shimoi
b,
Marion
Boudes
c,
Satoshi
Abe
b,
Fasséli
Coulibaly
c,
Susumu
Kitagawa
*a,
Hajime
Mori
d and
Takafumi
Ueno
*b
aGraduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: kitagawa@icems.kyoto-u.ac.jp
bGraduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Nagatsuta 4259, Midori-ku, Yokohama 224-8501, Japan. E-mail: tueno@bio.titech.ac.jp
cInfection and Immunity Program, Monash Biomedicine Discovery Institute and Department of Biochemistry and Molecular Biology, Monash University, Melbourne, VIC3800, Australia
dInsect Biomedical Research Center, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan
First published on 1st March 2016
Abstract
Photoactivatable CO releasing protein crystals were developed by immobilization of Mn carbonyl complexes in polyhedral crystals, which are spontaneously formed in insect cells. The photoactivatable CO release from the engineered protein crystals activates nuclear factor kappa B (NF-κB) upon stimulation by visible light irradiation with suppression of cytotoxicity of the Mn complex.
Carbon monoxide (CO) is one of several gaseous cellular-signaling molecules operating in living cells. CO mediates anti-inflammation and vasoactive response, and has been used to control immune responses during transplantation.1,2 CO signaling involves interactions with other gaseous signaling molecules such as nitric oxide and hydrogen sulfide, which are endogenously produced by the specific enzymes.3,4 Recently, the therapeutic applications of CO have attracted significant attention as a result of rapid development of a number of CO-releasing molecules (CORMs).5 Metal carbonyl complexes such as [Ru(CO)3Cl2]2 (CORM-2) and Ru(CO)3Cl(glycinate) (CORM-3), have been used to deliver CO into living cells.6 Synthetic and natural carrier molecules have been developed with the objective of providing safe and controlled delivery of CORMs into cells, tissues, and animals with effective CO-releasing properties.7–13 Moreover, a wide variety of transition metals and synthetic ligands have been developed for stimulus-induced CO release.14–16 These efforts represent promising strategies for providing controlled CO delivery.
Photoactivation is one of the most favorable external stimuli for CORMs. Other external stimuli include pH change, and enzymatic reactions.14–16 Photoactivatable CORMs (photoCORMs) are transition metal carbonyl complexes of Mn,17–19 Fe,,20–22 Ru,23 and Re,18,24 among others. Since Motterlini et al. reported that Mn2(CO)10 (CORM-1) can release CO with triggering by light irradiation,25 design of photoCORMs has been achieved by development of complexes based on organic ligands coordinating to Mn ions.17–19 Recently, Mascharak and co-workers have reported photoCORMs of Mn carbonyl complexes with polypyridine-based ligands to promote rapid CO release in the visible/near infrared region.26 For biotechnological applications of photoCORMs, cytotoxicity arising from free metal ions, organic ligands, or by the reaction of photoCORMs with light irradiation should be considered.27,28 One of the possible solutions is provided by incorporation of photoCORMs into biocompatible scaffolds to prevent release of metal ions or synthetic organic ligands.26 Nanoparticles, polymers, and porous materials have been utilized as molecular matrices for stable accumulation of photoCORMs.8,11,26,29,30 Schatzschneider and co-workers have utilized silica nanoparticles as photoCORM carriers.11 [Mn(CO)3(pqa)]ClO4 incorporated into Al-MCM-41 nanoparticles were found to promote vasorelaxation of rat aorta muscle rings through light-induced CO delivery.26 PhotoCORMs have been non-covalently and covalently embedded into polymers.30 However, their CO releasing properties have slow rates of ca. 20 min. Thus, improvement of carrier matrixes for photoCORMs is required for design of photoCORMs with rapid responses to stimuli.
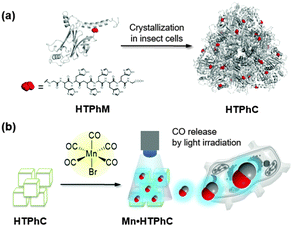
It has been recently reported that proteins and protein assemblies can serve as biocompatible carriers of CORMs.31–37 Composites of protein assemblies with CORMs can stably deliver CO into living cells and effectively release them to activate signal transduction.33–37 Protein crystals are also expected to be appropriate candidates for extracellular matrices (ECMs) for immobilization of CORMs because protein crystals represent precise protein assemblies in the solid state with inner pores that act as “solvent channels.”33,34 In general, when most of the crystals are stabilized by cross-linking, the crystals are utilized as vessels for preparation of metal nanoparticles or catalytic reactions by immobilization of metal compounds under various reaction conditions.38 It has been reported that cross-linked hen egg white lysozyme crystals can serve as extracellular matrices for CORM-2.33 However, inconvenient procedures which include crystallization and cross-linking are required to obtain the materials. To address this issue, we have used protein crystals, which are spontaneously synthesized in insect cells, as matrices for immobilizing CORMs.34 Polyhedral crystals (PhCs) are directly produced from polyhedrin monomer (PhM) expressed in insect cells after infection by cypovirus.39 PhCs are highly stable over a wide range of pH, temperature, and in organic solvents, and can be frozen or dried because their original function is to provide protective material to store replicated viruses produced during the viral infection cycle.39 PhCs have served as extracellular matrices for growth factors and CORMs due to their biocompatibility.34,40 In this article, we describe the immobilization of photoCORMs of Mn carbonyl complexes in PhCs, which can be used as ECM without cross-link treatment. To modulate the amounts of CO released from the composite, we increased the number of photoCORMs accumulated in PhCs by using a mutant of PhM with a hexa-histidine tag (His-tag) at its C-terminus (HTPhM). The crystal of HTPhM (HTPhC) containing Mn carbonyl complexes (Mn·HTPhC) has twice the number of Mn carbonyl complexes of WTPhC containing Mn carbonyl complexes (Mn·WTPhC). Mn·HTPhC was found to be capable of acting as an ECM that can release CO gas into living cells with a more rapid response than that of previously reported ECM-containing photoCORMs and activate nuclear factor kappa B (NF-κB) with visible light irradiation (Fig. 1).
HTPhCs were prepared as reported previously using an insect cell expression system of Spodoptera frugiperda 21 (Sf21).40 To immobilize Mn(CO) moieties into HTPhCs, HTPhCs (7.8 × 107 crystals) were soaked in 10 mM HEPES buffer (pH 7.0, 500 μL) containing 10% v/v acetonitrile and 1 mM Mn(CO)5Br. After gentle stirring for 24 hours at room temperature in the dark, the suspension was washed twice with Milli-Q water.
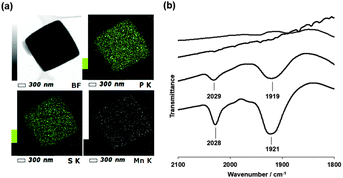
Fluorescence X-ray analysis of Mn·HTPhC showed that the number of Mn ions per HTPhM was 5.6 ± 1.2. The Mn complexes were fully dispersed in Mn·HTPhC as confirmed by a scanning transmission electron microscopy-energy dispersive X-ray spectrum (STEM-EDX) (Fig. 2a). The IR spectrum of Mn·HTPhC has two bands at 1921 and 2028 cm−1. This set of bands is assigned to the CO stretching vibrations of the fac-Mn(CO)3(H2O)2(His) moiety.41Mn·WTPhCs were also prepared under the same conditions. The number of Mn per WTPhM in Mn·WTPhC (2.6 ± 0.3) was found to be almost half the value of that of Mn·HTPhC with homogeneously-dispersed accumulation (Fig. S1, ESI†). The IR spectrum of Mn·WTPhC indicates the presence of Mn carbonyl moieties with a coordination structure similar to that of Mn·HTPhC (Fig. 2b).
The crystal structure of Mn·HTPhC was determined at a resolution of 1.8 Å (ESI†). The structure has no electron density for the extended sequence after Gly245, the carboxy-terminal residue in the wild-type protein. Moreover, an anomalous difference map does not identify densities assignable to Mn atoms in the structure although fluorescence X-ray analysis and STEM-EDX indicate the presence of Mn atoms in the composite. There is no electron density accounting for the presence of Mn in the crystal structure of Mn·WTPhC at a 1.7 Å resolution. These results suggest that the His-tag and Mn moieties can be oriented in several directions at the conjugation sites, as observed previously for Ru complexes conjugated in protein crystals.33
The photoactivatable CO-releasing properties of Mn·HTPhC were evaluated using the myoglobin (Mb) assay (Fig. 3 and Fig. S3, ESI†).7,25 The data processing steps correct for the uneven absorption of the CORM at the Q-bands in both deoxy-Mb and carbonmonoxy-Mb (MbCO) (Fig. 3a).25,42Mn·HTPhC (4.0 × 105 crystals with immobilized Mn ions determined by ICP-MS is 6.6 × 10−4 μmol) was dispersed in 1 mL of a PBS buffer (pH 7.4) containing deoxy-Mb (6.2 μM) and sodium dithionite (30 mM). The reaction was accompanied by light irradiation at 456 nm using a blue LED light (130 mW, 33.2 mW cm−2, Fig. S2, ESI†). The total released amount of CO per Mn of Mn·HTPhC with light irradiation was found to be 2.9 ± 0.4. Significantly less CO released from Mn·HTPhC was detected without irradiation (Fig. 3b). The amount of CO released is consistent with the coordination structure suggested by the IR spectrum of Mn·HTPhC and the Mn(CO)3Br(bpy) complex (Fig. 2b).43 The IR spectrum of Mn·HTPhC after the Mb assay with light irradiation indicates complete release of CO from Mn·HTPhC due to the absence of bands assignable to CO stretching (Fig. 2b, dotted line). The half-life (t1/2) of CO release from Mn·HTPhC was found to be 5.7 ± 0.3 min with light irradiation. The value is comparable to that of photoCORM immobilized in non-woven and polymer (Table S2, ESI†).10,44 The number of Mn ions per PhM retained in Mn·HTPhC after the Mb assay is 5.8 ± 1.0. This value is similar to the number of Mn ions determined before initiation of the assay (5.6 ± 1.2). These results suggest that Mn ions are retained in Mn·HTPhC after CO release. The amount of released of CO per Mn of Mn·WTPhC with light irradiation is 1.9 ± 0.1 with a t1/2 value of 6.9 ± 0.1 min. These results suggest that His-tag fragments of HTPhC contribute to acceleration of CO release in greater amounts and with a sharper response than WTPhC. Quantum yields of the precursor complex, Mn·HTPhC and Mn·WTPhC are 0.23, 0.047, and 0.013, respectively.
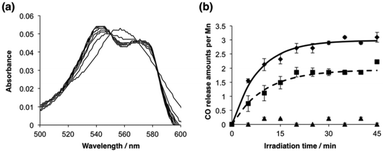 | ||
| Fig. 3 Mb assay of Mn·HTPhC and Mn·WTPhC: (a) time course of absorption spectral change of deoxy-Mb in a solution containing Mn·HTPhC with light irradiation, and (b) detection of CO release by plotting the concentration of MbCO. The absorption spectral change of MbCO was monitored for Mn·HTPhC with and without light irradiation (◆ and ▲, respectively), and Mn·WTPhC with light irradiation (■). All experiments were performed in 1 mL of 10 mM PBS buffer (pH 7.4) containing deoxy-Mb (6.2 μM) and Na2S2O4 (30 mM). The Mn concentrations of Mn·HTPhC and Mn·WTPhC determined by ICP-MS were 6.6 × 10−4 and 3.4 × 10−4 μmol, respectively, in the buffer solution. The suspended solution was exposed to the LED light at 456 nm. The spectra were recorded at 5 min intervals, and corrected at 550 nm to determine the conversions of deoxy-Mb to MbCO by using calculations reported previously.25,42 The experiments were performed in triplicate and the data represent mean ± SEM. | ||
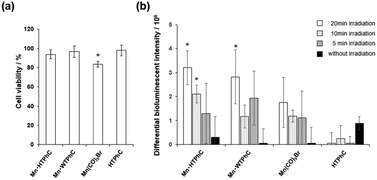
Mn·HTPhC was employed as an ECM for photoactivatable CO release to evaluate the activity of NF-κB by CO gas stress.45,46 When Mn·HTPhCs suspension was added to each well, most of Mn·HTPhCs interacted with the cell surface (Fig. S4, ESI†).33 The cell viability test assessed by the MTT assay indicates no cytotoxicity of Mn·HTPhC as well as other composites except for Mn(CO)5Br under light irradiation (Fig. 4a). Since it is well known that the amount and the rate of CO induction into living cells are essential for the activation of NF-κB,47 the activity of NF-κB is expected to be controlled by Mn·HTPhC in the presence or the absence of light irradiation.48 The activity of κB-Fluc transfected HEK293 cells (HEK293/κB-Fluc) cultured with Mn·HTPhC and TNF-α was assessed by examining the bioluminescence intensity of HEK293 cells after 12 hours incubation (Fig. 4b). Mn·HTPhC showed the activation of NF-κB almost comparable to Mn·WTPhC under light irradiation for 20 min. Only Mn·HTPhC had a significant activation of NF-κB with 10 min light irradiation. There were no significant differences among the composites with 5 min light irradiation or in the dark. The activation of NF-kB by Mn·HTPhC with 10 min light irradiation is expected to be caused by ca. 1.5-fold larger amount of CO from Mn·HTPhC than that of Mn·WTPhC (Fig. 3b), although Mn·HTPhC and Mn·WTPhC shows only slight difference on half-lives.35 Mn(CO)5Br showed less significant effect on the NF-κB activation. This result could be due to cytotoxicity of free Mn species after the CO release with the light irradiation because the complex did not show the toxicity before light irradiation (Fig. 4a and Fig. S5, ESI†). Thus, these results indicate that Mn·HTPhC can safely release CO to activate NF-κB by suppressing the cytotoxicity of the Mn(CO) moieties in the crystals.
In conclusion, light-triggered CO-releasing ECMs were constructed by immobilizing Mn(CO) moieties in PhCs. When a His-tag fragment is fused to the C-terminus of PhM, the resulting composite HTPhC gains the ability to accumulate twice the number of Mn moieties relative to the unmodified WTPhC. Moreover, Mn·HTPhC releases a stoichiometric amount of CO molecules per Mn ion. The release effectively contributes to activation of NF-κB by suppressing the cytotoxicity of the precursor complex, Mn(CO)5Br. These results suggest that photoactivatable CO release from engineered protein crystals will be adaptable to provide information regarding cellular events involving CO gas. Since CO release is expected to be regulated at a specific time point during the course of cellular proliferation, we are investigating the detailed mechanisms of CO biology using these composites.
The authors thank Prof. S. Kizaka-Kondoh and Dr T. Kuchimaru, and Dr H. Ohtani (Tokyo Institute of Technology, Japan) for their discussions. Parts of this work were supported by the Funding Program for Next-Generation World-Leading Researchers (grant number LR019 for T. U.) for Scientific Research on Innovative Areas (26102513 for T. U. and 15K07794 for H. M.) from Ministry of Education, Culture, Sports, Science and Technology, and Research Fellowship for Young Scientists (for H. T.) from the Japan Society for the Promotion of Science.
References
- R. Motterlini and L. E. Otterbein, Nat. Rev. Drug Discovery, 2010, 9, 728–743 CrossRef CAS PubMed.
- T. R. Johnson, B. E. Mann, J. E. Clark, R. Foresti, C. J. Green and R. Motterlini, Angew. Chem., Int. Ed., 2003, 42, 3722–3729 CrossRef CAS PubMed.
- B. E. Mann and R. Motterlini, Chem. Commun., 2007, 4197–4208 RSC.
- M. Kajimura, R. Fukuda, R. M. Bateman, T. Yamamoto and M. Suematsu, Antioxid. Redox Signaling, 2010, 13, 157–192 CrossRef CAS PubMed.
- U. Schatzschneider, Br. J. Pharmacol., 2015, 172, 1638–1650 CrossRef CAS PubMed.
- J. E. Clark, P. Naughton, S. Shurey, C. J. Green, T. R. Johnson, B. E. Mann, R. Foresti and R. Motterlini, Circ. Res., 2003, 93, E2–E8 CrossRef CAS PubMed.
- U. Hasegawa, A. J. van der Vlies, E. Simeoni, C. Wandrey and J. A. Hubbell, J. Am. Chem. Soc., 2010, 132, 18273–18280 CrossRef CAS PubMed.
- H. Yin, J. Fang, L. Liao, H. Nakamura and H. Maeda, J. Controlled Release, 2014, 187, 14–21 CrossRef CAS PubMed.
- P. Peng, C. Wang, Z. Shi, V. K. Johns, L. Ma, J. Oyer, A. Copik, R. Igarashi and Y. Liao, Org. Biomol. Chem., 2013, 11, 6671–6674 CAS.
- P. C. Kunz, H. Meyer, J. Barthel, S. Sollazzo, A. M. Schmidt and C. Janiak, Chem. Commun., 2013, 49, 4896–4898 RSC.
- G. Doerdelmann, H. Pfeiffer, A. Birkner and U. Schatzschneider, Inorg. Chem., 2011, 50, 4362–4367 CrossRef PubMed.
- G. Doerdelmann, T. Meinhardt, T. Sowik, A. Krueger and U. Schatzschneider, Chem. Commun., 2012, 48, 11528–11530 RSC.
- A. E. Pierri, P.-J. Huang, J. V. Garcia, J. G. Stanfill, M. Chui, G. Wu, N. Zheng and P. C. Ford, Chem. Commun., 2015, 51, 2072–2075 RSC.
- U. Schatzschneider, Inorg. Chim. Acta, 2011, 374, 19–23 CrossRef CAS.
- R. D. Rimmer, A. E. Pierri and P. C. Ford, Coord. Chem. Rev., 2012, 256, 1509–1519 CrossRef CAS.
- M. A. Gonzales and P. K. Mascharak, J. Inorg. Biochem., 2014, 133, 127–135 CrossRef CAS PubMed.
- J. Niesel, A. Pinto, H. W. P. N'Dongo, K. Merz, I. Ott, R. Gust and U. Schatzschneider, Chem. Commun., 2008, 1798–1800 RSC.
- P. C. Kunz, W. Huber, A. Rojas, U. Schatzschneider and B. Spingler, Eur. J. Inorg. Chem., 2009, 5358–5366 CrossRef CAS.
- M. A. Gonzalez, M. A. Yim, S. Cheng, A. Moyes, A. J. Hobbs and P. K. Mascharak, Inorg. Chem., 2012, 51, 601–608 CrossRef CAS PubMed.
- R. Kretschmer, G. Gessner, H. Gorls, S. H. Heinemann and M. Westerhausen, J. Inorg. Biochem., 2011, 105, 6–9 CrossRef CAS PubMed.
- A. J. Atkin, I. J. S. Fairlamb, J. S. Ward and J. M. Lynam, Organometallics, 2012, 31, 5894–5902 CrossRef CAS.
- C. S. Jackson, S. Schmitt, Q. P. Dou and J. J. Kodanko, Inorg. Chem., 2011, 50, 5336–5338 CrossRef CAS PubMed.
- C. Bischof, T. Joshi, A. Dimri, L. Spiccia and U. Schatzschneider, Inorg. Chem., 2013, 52, 9297–9308 CrossRef CAS PubMed.
- A. E. Pierri, A. Pallaoro, G. Wu and P. C. Ford, J. Am. Chem. Soc., 2012, 134, 18197–18200 CrossRef CAS PubMed.
- R. Motterlini, J. E. Clark, R. Foresti, P. Sarathchandra, B. E. Mann and C. J. Green, Circ. Res., 2002, 90, E17–E24 CrossRef CAS PubMed.
- M. A. Gonzales, H. Han, A. Moyes, A. Radinos, A. J. Hobbs, N. Coombs, S. R. J. Oliver and P. K. Mascharak, J. Mater. Chem. B, 2014, 2, 2107–2113 RSC.
- S. Garcia-Gallego and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2014, 53, 9712–9721 CrossRef CAS PubMed.
- S. H. Heinemann, T. Hoshi, M. Westerhausen and A. Schiller, Chem. Commun., 2014, 50, 3644–3660 RSC.
- H. Inaba, K. Fujita and T. Ueno, Biomater. Sci., 2015, 3, 1423–1438 RSC.
- N. E. Bruckmann, M. Wahl, G. J. Reiss, M. Kohns, W. Watjen and P. C. Kunz, Eur. J. Inorg. Chem., 2011, 4571–4577 CrossRef.
- I. S. Albuquerque, H. F. Jeremias, M. Chaves-Ferreira, D. Matak-Vinkovic, O. Boutureira, C. C. Romao and G. J. L. Bernardes, Chem. Commun., 2015, 51, 3993–3996 RSC.
- M. Chaves-Ferreira, I. S. Albuquerque, D. Matak-Vinkovic, A. C. Coelho, S. M. Carvalho, L. M. Saraiva, C. C. Romao and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2015, 54, 1172–1175 CrossRef CAS PubMed.
- H. Tabe, K. Fujita, S. Abe, M. Tsujimoto, T. Kuchimaru, S. Kizaka-Kondoh, M. Takano, S. Kitagawa and T. Ueno, Inorg. Chem., 2015, 54, 215–220 CrossRef CAS PubMed.
- H. Tabe, T. Shimoi, K. Fujita, S. Abe, H. Ijiri, M. Tsujimoto, T. Kuchimaru, S. Kizaka-Kondo, H. Mori, S. Kitagawa and T. Ueno, Chem. Lett., 2015, 44, 342–344 CrossRef CAS.
- K. Fujita, Y. Tanaka, T. Sho, S. Ozeki, S. Abe, T. Hikage, T. Kuchimaru, S. Kizaka-Kondoh and T. Ueno, J. Am. Chem. Soc., 2014, 136, 16902–16908 CrossRef CAS PubMed.
- K. Fujita, Y. Tanaka, S. Abe and T. Ueno, Angew. Chem., Int. Ed., 2015, 55, 1056–1060 CrossRef PubMed.
- H. Inaba, N. J. M. Sanghamitra, K. Fujita, T. Sho, T. Kuchimaru, S. Kitagawa, S. Kizaka-Kondoh and T. Ueno, Mol. BioSyst., 2015, 11, 3111–3118 RSC.
- S. Abe and T. Ueno, RSC Adv., 2015, 5, 21366–21375 RSC.
- F. Coulibaly, E. Chiu, K. Ikeda, S. Gutmann, P. W. Haebel, C. Schulze-Briese, H. Mori and P. Metcalf, Nature, 2007, 446, 97–101 CrossRef CAS PubMed.
- H. Ijiri, F. Coulibaly, G. Nishimura, D. Nakai, E. Chiu, C. Takenaka, K. Ikeda, H. Nakazawa, N. Hamada, E. Kotani, P. Metcalf, S. Kawamata and H. Mori, Biomaterials, 2009, 30, 4297–4308 CrossRef CAS PubMed.
- M. Razavet, V. Artero, C. Cavazza, Y. Oudart, C. Lebrun, J. C. Fontecilla-Camps and M. Fontecave, Chem. Commun., 2007, 2805–2807 RSC.
- A. J. Atkin, J. M. Lynam, B. E. Moulton, P. Sawle, R. Motterlini, N. M. Boyle, M. T. Pryce and I. J. Fairlamb, Dalton Trans., 2011, 40, 5755–5761 RSC.
- P. Govender, S. Pai, U. Schatzschneider and G. S. Smith, Inorg. Chem., 2013, 52, 5470–5478 CrossRef CAS PubMed.
- C. Bohlender, S. Gläser, M. Klein, J. Weisser, S. Thein, U. Neugebauer, J. Popp, R. Wyrwa and A. Schiller, J. Mater. Chem. B, 2014, 2, 1454–1463 RSC.
- H. S. Kim, P. A. Loughran, P. K. Kim, T. R. Billiar and B. S. Zuckerbraun, Biochem. Biophys. Res. Commun., 2006, 344, 1172–1178 CrossRef CAS PubMed.
- H. S. Kim, P. A. Loughran, J. Rao, T. R. Billiar and B. S. Zuckerbraun, Am. J. Physiol.: Gastrointest. Liver Physiol., 2008, 295, G146–G152 CrossRef CAS PubMed.
- S. Chlopicki, M. Lomnicka, A. Fedorowicz, E. Grochal, K. Kramkowski, A. Mogielnicki, W. Buczko and R. Motterlini, Naunyn-Schmiedeberg's Arch. Pharmacol., 2012, 385, 641–650 CrossRef CAS PubMed.
- A. R. Brasier, J. E. Tate and J. F. Habener, Biotechniques, 1989, 7, 1116–1122 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experiments, structures and crystallographic dates; Fig. S1–S4; Tables S1. See DOI: 10.1039/c5cc10440h |
| This journal is © The Royal Society of Chemistry 2016 |