Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Studying the effects of asymmetry on the bending rigidity of lipid membranes formed by microfluidics†
K.
Karamdad
ab,
R. V.
Law
ab,
J. M.
Seddon
ab,
N. J.
Brooks
ab and
O.
Ces
*ab
aDepartment of Chemistry, Imperial College London, Exhibition Road, London, SW7 2AZ, UK. E-mail: o.ces@imperial.ac.uk
bInstitute of Chemical Biology, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
First published on 11th March 2016
Abstract
In this article we detail a robust high-throughput microfluidic platform capable of fabricating either symmetric or asymmetric giant unilamellar vesicles (GUVs) and characterise the mechanical properties of their membranes.
Despite the importance of lipid asymmetry in biology1–7 our understanding of its role and mode of action remains in its infancy, with technological limitations associated with manufacturing asymmetric membranes being one of the main obstacles.
GUVs are cell-sized aqueous spheres enclosing an internal aqueous environment bounded by a phospholipid bilayer. They can exhibit the full scope of fundamental traits of biological membranes, such as bilayer size, curvature and shape, lamellarity, asymmetry and the capacity to accommodate functional transmembrane proteins.
They have been used across a wide scale of applications, notably, as a chassis for artificial cells and protocell systems.8–14 They have also been used recently to demonstrate that mechanical properties such as the bending rigidity, which characterises the ability of membranes to bend under low stress, are significantly affected by chain length,15,16 headgroup type,17 presence of proteins18 and recently, most intriguingly through the presence of lipid asymmetry in model membrane systems.19
Sophisticated methods to manufacture synthetic vesicles have been developed in recent years with microfluidic platforms introducing finer control over certain vesicle structural parameters such as size, lamellarity and composition.20–24 However, most microfluidic methods have failed to address the incorporation of membrane asymmetry which is an oversight as they are an ideal platform with which to study membrane asymmetry. Non-microfluidic strategies to generate asymmetric giant vesicles have emerged, most notably, the emulsion phase transfer method although this approach though is low-throughput and non-continuous.25,26
In this paper, we present a microfluidic platform for the generation of asymmetric GUVs. We have validated the asymmetry of the vesicles by selectively performing rapid Cu-free click chemistry in the membrane of these systems.27–29 We have also used this system in conjunction with fluctuation analysis to determine the bending rigidity of both symmetric and asymmetric vesicles generated by microfluidics.
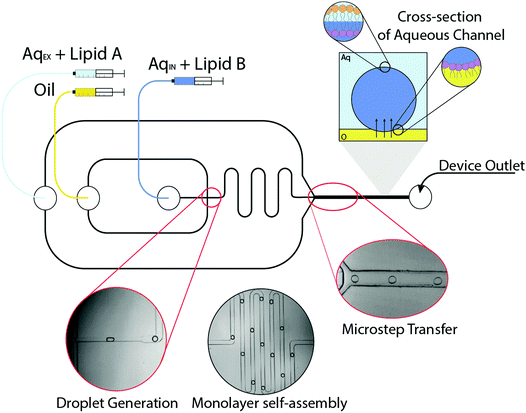
The microfluidic method for generating asymmetric vesicles exploits a strategy previously used to manufacture symmetric systems.23 The device was fabricated using standard lithography protocols, although a significant modification is the double-layer fabrication that is required to form the ‘microstep’.30 Water-in-oil (W/O) microdroplets were generated using a flow focussing mechanism in microfluidic channels (Fig. 1). A dispersed aqueous phase containing 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, 5 mg mL−1) was sheared by a continuous squalene oil phase to form emulsions, the flow rates were 0.1 and 1.0 μL min−1 respectively. Squalene was used as the oil phase due to its preferential wettability with regards to forming stable droplets in PDMS channels.31 As can be seen from Fig. 1A, water-in-oil droplets generated at the flow focussing junction were stabilised by the self-assembly of a POPC monolayer, which form spontaneously using the ‘lipid in’ approach.32 The POPC monolayer-stabilised droplets are transported through a continuous oil meander channel (Fig. 1B) and then transferred across a microstep (change in channel depth from 50 μm to 100 μm), into a deeper water-containing channel, which facilitated phase transfer (from oil to water) (Fig. 1C). As the POPC monolayer-stabilised droplets are transferred through the interface they become bounded by a second lipid monolayer coating of 2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, 3 mg mL−1) in the external aqueous phase (from small vesicles/micelles), which was pumped in at a flow rate of ∼80.0 μL min−1. As the lipids present in the aqueous phases are different (AqEX + lipid A vs. AqIN + lipid B in Fig. 1) this leads to the construction of an asymmetric vesicle.
The resulting vesicles were then collected from the PDMS device via PTFE tubing (1 mm outer diameter) and studied in PDMS columns. To demonstrate that the giant vesicles produced were asymmetric we performed a series of click chemistry experiments to obtain specific information about the composition of each leaflet in the GUV membranes. Strain-promoted alkyne–azide click chemistry (SPAAC) describes the reaction between a strained alkyne and an azide to afford a triazole product (Fig. 2). This type of bioorthogonal chemistry has been applied across various biological systems such as cultured cells, live zebrafish and mice.28,29,33,34 The advantages being the enhanced rates of reaction and the absence of a cytotoxic copper catalyst typically associated with traditional click reactions.35
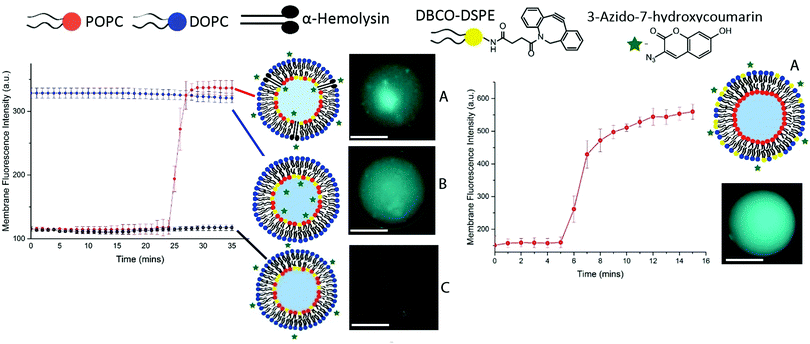
Vesicles were generated in the same manner as previously outlined36 and were doped with head-group-labelled alkyne lipid, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[dibenzocyclooctyl(polyethylene glycol)-2000], (DSPE-DBCO, 5 mol%) on the inner leaflet (Fig. 2, left) and outer leaflet (Fig. 2, right).
A fluorogenic azide (3-azido-7-hydroxycoumarin, 5 mM), which gives a fluorescence signal once clicked with a strained alkyne, was used in various permutations in order to characterise asymmetry in the vesicles produced (Fig. 2).
Fig. 2 illustrates the various permutations of the click reaction that we performed on DBCO-tagged inner leaflet vesicles. (A) Vesicles tagged with DBCO on the inner leaflet, where fluorogenic azide was added externally to the vesicles as well as protein pore αHL. Here, we observed an increase in the fluorescent triazole click product after 25 minutes due to the translocation of azide across the bilayer into the vesicle. (B) The reaction between DBCO on the inner leaflet and fluorogenic azide encapsulated internally in the vesicles, affording the fluorescent triazole product from the outset. (C) Vesicles tagged with DBCO on the inner leaflet where the fluorogenic azide has been added externally to the vesicles and resulted in the formation of no fluorescent product. Fig. 2 (right) illustrates the reaction between the DBCO-tagged outer leaflet vesicles and fluorogenic azide added externally.
The azide is added after 5 minutes which results in the formation of the fluorescent triazole product on the outer leaflet of the vesicle. The results of the click chemistry assay outlined in Fig. 2 demonstrate the successful manufacture of asymmetric vesicles using a microfluidic approach. Using the ‘lipid in’ approach for assembling lipid monolayers we have shown that we are able to incorporate the DBCO-lipid in either membrane leaflet. By reconstituting αHL we have shown that we can instigate the translocation of the azide into the core of the vesicles and observe the reaction of the two click reagents to afford a fluorescent signal.
To determine GUV bending rigidities we employed the fluctuation analysis technique as described in further detail in the ESI,† SI 3.
Previously we reported that the value for κ for symmetric microfluidic vesicles composed purely of 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) was 1.29 ± 0.37 × 10−19 J, which demonstrated that GUVs produced by microfluidics exhibited analogous mechanical properties to ones made traditionally by electroformation.23
With the knowledge that there was no significant discrepancy in studying the mechanical properties of membranes assembled by microfluidics and from electroformation, we have investigated the effects of introducing asymmetry upon the bending rigidities of microfluidic GUVs. We performed fluctuation analysis on 10 vesicles of each lipid composition; we studied two symmetric two-component compositions: DPhPC/POPC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and DOPC/POPC (1
1) and DOPC/POPC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
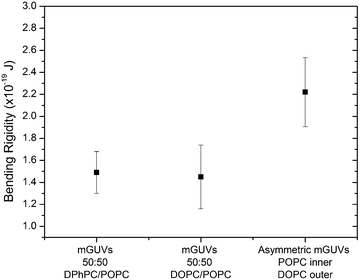
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as well as an asymmetric POPC/DOPC system with pure POPC in the inner leaflet and DOPC in the outer leaflet (Fig. 3). This asymmetric composition was chosen in accordance to the work done by Elani et al.,19 who reported κ in asymmetric GUV compositions of POPC and DOPC, and found that there was little discrepancy in the rigidity of either permutation of POPC or DOPC on either membrane leaflet. The bending rigidity for the symmetric compositions of DPhPC/POPC (1
1) as well as an asymmetric POPC/DOPC system with pure POPC in the inner leaflet and DOPC in the outer leaflet (Fig. 3). This asymmetric composition was chosen in accordance to the work done by Elani et al.,19 who reported κ in asymmetric GUV compositions of POPC and DOPC, and found that there was little discrepancy in the rigidity of either permutation of POPC or DOPC on either membrane leaflet. The bending rigidity for the symmetric compositions of DPhPC/POPC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and DOPC/POPC (1
1) and DOPC/POPC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were found to be 1.49 ± 0.19 × 10−19 J and 1.45 ± 0.29 × 10−19 J respectively. These results were concordant with previously reported values for symmetric compositions of two component lipid GUVs.19 The value obtained for the asymmetric GUVs was 2.22 ± 0.31 × 10−19 J, this result agrees with what was expected of an asymmetric membrane from previous literature.19
1) were found to be 1.49 ± 0.19 × 10−19 J and 1.45 ± 0.29 × 10−19 J respectively. These results were concordant with previously reported values for symmetric compositions of two component lipid GUVs.19 The value obtained for the asymmetric GUVs was 2.22 ± 0.31 × 10−19 J, this result agrees with what was expected of an asymmetric membrane from previous literature.19
The value can be rationalised if we consider the individual spontaneous curvatures of the POPC and DOPC lipids. Typically, in a symmetric system we only consider the spontaneous curvature of a single lipid (even in a two component mixture). If we interpret the asymmetric membrane as two individual monolayers, where the outer and inner monolayers can be assumed to have independent spontaneous curvature values, we can approximate the curvature elastic energy (gcB). This can be determined by adding the spontaneous curvatures for each lipid in either monolayer along with the bending modulus for each monolayer.37,38 It has been previously reported that a symmetric bilayer is a lower energy system due to less curvature elastic stress and therefore we would expect the bending rigidity to be a lower value than in an asymmetric system.19 Our results agreed with this hypothesis and with what was expected of the physical nature of a lipid membrane.
The implication of our findings suggests that asymmetry significantly affects the mechanical properties of membranes, we would expect that this will have significant consequences when studying interactions between membranes and membrane proteins such as the mechanosensitive channel, MscL.39,40
By employing the ‘lipid in’ approach to monolayer formation, we have shown that asymmetric GUVs can be generated using a microfluidic method. Asymmetry was confirmed in the vesicles through a series of simple, yet elegant, click chemistry reactions on both the inner and outer leaflet of the bilayer.
By combining microfluidic technology and fluctuation analysis, we have been able to demonstrate that asymmetry has a significant impact on membrane bending rigidity. This is building upon initial work reported by Elani et al.,19 where asymmetry was shown to cause an increase in bending rigidity on vesicles built by emulsion phase transfer. Significantly, we have shown that miniaturising the vesicle formation process using microfluidics affords the same outcome and agrees with these findings.
Having the means to generate asymmetric GUVs in high throughput with a user-defined composition is a significant development towards furthering our understanding of the effects of asymmetry in biological systems. Previous literature has already hinted at a significant link between asymmetry and phenomena such as protein folding,41,42 membrane protein behaviour and gating39,40 as well as membrane-associated proteins regulated by membrane mechanics.43,44 The mechanism of cell endo- and exocytosis has also been shown to be effected by mechanical changes across the membrane which are potentially brought about by asymmetry.45,46
This work was supported by EPSRC via grant EP/J017566/1 and an EPSRC Centre for Doctoral Training Studentship EP/K502856/1 from the Institute of Chemical Biology (Imperial College London) awarded to KK. All data created during this research are openly available from Imperial College London, please see contact details at http://www.imperial.ac.uk/membranebiophysics.
Notes and references
- R. F. A. Zwaal and A. J. Schroit, J. Am. Soc. Hematol., 1999, 93, 2143–2148 Search PubMed.
- S. Manno, Y. Takakuwa and N. Mohandas, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 1943–1948 CrossRef CAS PubMed.
- B. Fadeel and D. Xue, Crit. Rev. Biochem. Mol. Biol., 2009, 44, 264–277 CrossRef CAS PubMed.
- F. X. Contreras, L. Sánchez-Magraner, A. Alonso and F. M. Goñi, FEBS Lett., 2010, 584, 1779–1786 CrossRef CAS PubMed.
- B. Fadeel and D. Xue, Cell Death Differ., 2006, 13, 360–362 CrossRef CAS PubMed.
- D. L. Daleke, J. Biol. Chem., 2007, 282, 821–825 CrossRef CAS PubMed.
- Z. Ma, Z. Liu and X. Huang, Genetics, 2012, 190, 1299–1308 CrossRef CAS PubMed.
- Y. Elani, R. V Law and O. Ces, Nat. Commun., 2014, 5, 1–5 Search PubMed.
- K. Kurihara, M. Tamura, K. Shohda, T. Toyota, K. Suzuki and T. Sugawara, Nat. Chem., 2011, 3, 775–781 CrossRef CAS PubMed.
- Y. Elani, R. V. Law and O. Ces, Phys. Chem. Chem. Phys., 2015, 17, 15534–15537 RSC.
- Y. Elani, A. Gee, R. V. Law and O. Ces, Chem. Sci., 2013, 4, 3332 RSC.
- P. M. Gardner, K. Winzer and B. G. Davis, Nat. Chem., 2009, 1, 377–383 CrossRef CAS PubMed.
- K. Charalambous, P. J. Booth, R. Woscholski, J. M. Seddon, R. H. Templer, R. V Law, L. M. C. Barter and O. Ces, J. Am. Chem. Soc., 2012, 134, 5746–5749 CrossRef CAS PubMed.
- P. Walde, BioEssays, 2010, 32, 296–303 CrossRef CAS PubMed.
- W. Rawicz, K. C. Olbrich, T. McIntosh, D. Needham and E. Evans, Biophys. J., 2000, 79, 328–339 CrossRef CAS PubMed.
- L. Fernandez-Puente, I. Bivas, M. D. Mitov and P. Méléard, Europhys. Lett., 1994, 28, 181–186 CrossRef.
- B. Brüning, R. Stehle, P. Falus and B. Farago, Eur. Phys. J. E: Soft Matter Biol. Phys., 2013, 36, 77 CrossRef PubMed.
- P. Girard, J. Prost and P. Bassereau, Phys. Rev. Lett., 2005, 94, 2–5 CrossRef PubMed.
- Y. Elani, S. Purushothaman, P. J. Booth, J. M. Seddon, N. J. Brooks, R. V. Law and O. Ces, Chem. Commun., 2015, 51, 6976–6979 RSC.
- S. Matosevic, BioEssays, 2012, 34, 992–1001 CrossRef CAS PubMed.
- S. Matosevic and B. M. Paegel, J. Am. Chem. Soc., 2011, 133, 2798–2800 CrossRef CAS PubMed.
- A. deMello and D. van Swaay, Lab Chip, 2013, 13, 752–767 RSC.
- K. Karamdad, R. V. Law, J. M. Seddon, N. J. Brooks and O. Ces, Lab Chip, 2015, 15, 557–562 RSC.
- S. Matosevic and B. M. Paegel, Nat. Chem., 2013, 5, 958–963 CrossRef CAS PubMed.
- S. Pautot, B. J. Frisken and D. a. Weitz, Langmuir, 2003, 19, 2870–2879 CrossRef CAS.
- P. C. Hu, S. Li and N. Malmstadt, ACS Appl. Mater. Interfaces, 2011, 3, 1434–1440 CAS.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed.
- X. Zhang and Y. Zhang, Molecules, 2013, 18, 7145–7159 CrossRef CAS PubMed.
- B. M. Zeglis, K. K. Sevak, T. Reiner, P. Mohindra, S. D. Carlin, P. Zanzonico, R. Weissleder and J. S. Lewis, J. Nucl. Med., 2013, 54, 1389–1396 CrossRef CAS PubMed.
- P. J. Hung, P. J. Lee, P. Sabounchi, N. Aghdam, R. Lin and L. P. Lee, Lab Chip, 2005, 5, 44–48 RSC.
- W. Li, Z. Nie, H. Zhang, C. Paquet, M. Seo, P. Garstecki and E. Kumacheva, Langmuir, 2007, 23, 8010–8014 CrossRef CAS PubMed.
- W. L. Hwang, M. Chen, B. Cronin, M. a Holden and H. Bayley, J. Am. Chem. Soc., 2008, 130, 5878–5879 CrossRef CAS PubMed.
- S. Cavalli, A. R. Tipton, M. Overhand and A. Kros, Chem. Commun., 2006, 3193–3195 RSC.
- F. S. Hassane, B. Frisch and F. Schuber, Bioconjugate Chem., 2006, 17, 849–854 CrossRef CAS PubMed.
- L. Liang and D. Astruc, Coord. Chem. Rev., 2011, 255, 2933–2945 CrossRef CAS.
- K. Karamdad, R. V. Law, J. M. Seddon, N. J. Brooks and O. Ces, Lab Chip, 2015, 15, 557–562 RSC.
- N. J. Brooks, O. Ces, R. H. Templer and J. M. Seddon, Chem. Phys. Lipids, 2011, 164, 89–98 CrossRef CAS PubMed.
- B. Kollmitzer, P. Heftberger, M. Rappolt and G. Pabst, Soft Matter, 2013, 9, 10877–10884 RSC.
- O. H. Samuli Ollila, M. Louhivuori, S. J. Marrink and I. Vattulainen, Biophys. J., 2011, 100, 1651–1659 CrossRef CAS PubMed.
- E. Perozo, A. Kloda, D. M. Cortes and B. Martinac, Nat. Struct. Biol., 2002, 9, 696–703 CrossRef CAS PubMed.
- P. J. Booth, R. H. Templer, W. Meijberg, S. J. Allen, a. R. Curran and M. Lorch, Crit. Rev. Biochem. Mol. Biol., 2001, 36, 501–603 CrossRef CAS PubMed.
- M. L. Riley, B. a Wallace, S. L. Flitsch and P. J. Booth, Biochemistry, 1997, 36, 192–196 CrossRef CAS PubMed.
- G. S. Attard, R. H. Templer, W. S. Smith, A. N. Hunt and S. Jackowski, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9032–9036 CrossRef CAS PubMed.
- G. S. Attard, A. N. Hunt, S. Jackowski, M. Baciu, S. C. Sebai, X. Mulet, J. A. Clarke, R. V Law, C. Plisson, C. A. Parker, A. Gee, O. Ces and R. H. Templer, Biochem. Soc. Trans., 2007, 35, 498–501 CrossRef PubMed.
- Z. Omran, P. Williams and C. Rauch, Symmetry, 2015, 7, 1780–1787 CrossRef.
- B. Hissa and L. O. Andrade, et al. , PLoS One, 2013, 8, 1–17 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc10307j |
| This journal is © The Royal Society of Chemistry 2016 |