Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Determining the structural stability of UiO-67 with respect to time: a solid-state NMR investigation†
M. C.
Lawrence
a,
C.
Schneider
b and
M. J.
Katz
*a
aDepartment of Chemistry, Memorial University of Newfoundland, St. John's, Newfoundland and Labrador, Canada. E-mail: mkatz@mun.ca
bC-CART NMR Facility, CREAIT, Memorial University of Newfoundland, Canada
First published on 4th February 2016
Abstract
The stability of UiO-67 has been questioned for some time. We have used solid-state NMR to investigate the temporal stability of this MOF. Proper activation is necessary to achieve optimal surface area. However, even with proper activation, the long-term (30+ days) fate of UiO-67 is hydrolysis of the linker-metal bonds and, ultimately, pore collapse.
Metal–organic frameworks (MOFs) are porous materials formed via coordination of bridging organic ligands (linkers) with inorganic metal cations/clusters (nodes). With judicious choice of these components, MOFs with applications in gas-storage,1 chemical separations,2 light-harvesting,3 sensing,2b,4 and catalysis5 have been realized. One family of MOFs which are becoming ubiquitous in these applications is the Zr-cluster-containing family of MOFs, including, but not limited to, UiOs,6 NU-1000,7 PCN-222,8 and MOF-808.9 The interest in these MOFs stems from their thermal, chemical, and mechanic stability making them ideal for many applications.6d,10
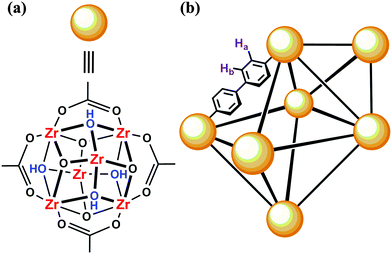
With respect to both anecdotal evidence as well as literature precedence, UiO-67 (Fig. 1) has had a precarious history. DeCoste et al. demonstrated that the internal surface area (SA) of UiO-67 decreased from 2145 m2 g−1 to 10 m2 g−1 after the MOF was exposed to 90% relative humidity. Similarly, when soaked in water, UiO-67 was found to be unstable; powder X-ray diffraction data (pxrd) indicated the presence of ZrO2.11 Although the instability is attributed to hydrolysis of the bonds between the linker and node, FTIR data showed no vibrational changes to corroborate this.
In a related manuscript, Mondloch et al. have proposed an alternate hypothesis. When UiO-67 was activated (i.e., the process of removing solvent from the porous frameworks) from water, then there was no notable porosity remaining. However, if water in the pore was replaced with acetone prior to activation, then the porosity remained; similar results were obtained when UiO-67 was boiled in water prior to solvent exchange. Thus, rather than an inherent instability in the MOF it was proposed that capillary-force driven collapse, due to improper activation, is responsible for the proposed instability.12
Given the utility of UiO-67,5b,6h,13 we were interested in further probing its stability. Specifically, we are interested in investigating the long-term stability of UiO-67 with respect to time. We turned our attention to solid-state NMR (SS-NMR) as a probe for the potential structural changes that occur within this MOF. Unlike pxrd, which is sensitive to crystalline materials containing high Z nuclei, SS-NMR is equally sensitive to both amorphous and crystalline materials. Furthermore, SS-NMR has the potential to independently report on each nucleus.
UiO-67 was synthesized by the method of Katz et al. (See ESI,† for further details).6a UiO-67 was subsequently filter-dried (ca. 1 h). The samples of DeCoste et al.11 were similarly filtered.
As expected, despite the apparently dry UiO-67, the SS-NMR indicates that freshly-prepared UiO-67 (Fig. 2 red trace) shows remanence of methanol (3.05 ppm, 4.65 ppm) within the pores of the MOF; the remaining resonances at 7.82 and 8.29 ppm belong to the biphenyl protons (Fig. 1 Ha and Hb).
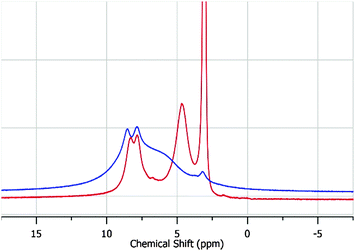 | ||
| Fig. 2 (red) 1H-NMR of UiO-67 immediately after vacuum filtration. (blue) 1H-NMR of UiO-67 6 days after vacuum filtration. The corresponding 13C-NMRs can be seen in Fig. S2 (ESI†). | ||
When freshly-prepared UiO-67 was left out for 6 days, the SS-NMR indicated that the majority of the methanol signals were greatly diminished.14 However, a new broad-featureless resonance upfield of the linker protons (6.29 ppm) with a concomitant broad resonance buried at 8.51 ppm was observed. This feature is indicative of the formation of an amorphous material. Concomitantly, the Brunauer–Emmett–Teller (BET) SA of the 6-day old sample was a mere 500 m2 g−1 (Fig. S1 in the ESI†); this is in contrast with the SA of freshly-prepared and thermally activated UiO-67 which exhibited a BET SA of 2000 m2 g−1 (Fig. S1 in the ESI†). These results are consistent with the work by DeCoste et al.11
In order to further probe whether hydrolysis of the Zr-carboxylate bonds or capillary-force driven collapse is the culprit, we repeated the experiment with UiO-67 which was solvent exchanged (4 days) and subsequently filtered and thermally activated. As illustrated in Fig. 3, there are three distinct regions at ca. 0 ppm, 2.5 ppm, and 7.5 ppm; the latter two resonances have been attributed to the linker (7.5 ppm) and the bridging hydroxides (Fig. 1) on the node (2.5 ppm).15 The remaining resonance at 0 ppm, which is only slightly visible in the spectra by Dolbecq et al.,15 we attribute to linker deficiencies (i.e., defect sites comprised of Zr-bound OH and H2O moieties on the Zr6(OH)4O412+ node) within the porous framework.6a,h,16
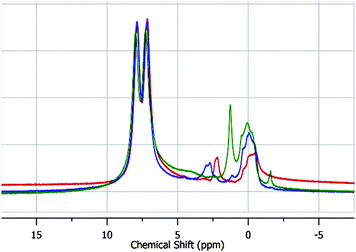 | ||
| Fig. 3 (red) 1H-NMR of UiO-67 immediately after thermal activation from methanol. (blue) 1H-NMR of UiO-67 4 days after thermal activation. (green) 1H-NMR of UiO-67 1 month after thermal activation. The corresponding 13C-NMRs can be seen in Fig. S3 (ESI†). | ||
Unlike the filter-dry sample which contains pore-bound solvent (Fig. 2), over the course of a month, the BET SA of activated UiO-67 merely decreased to 1500 m2 g−1 (Fig. S1 in the ESI†). The SS-NMR (Fig. 3) shows nearly no evidence for the broad featureless hump in Fig. 2 suggesting that the origin of the decrease in SA for filter-dried UiO-67 (Fig. 2) is due to capillary-force driven collapse.12
As a function of time however, the spectra in Fig. 3 show that the μ3-OH resonance (2.5 ppm) shifts with a concomitant increase in the intensity of the defect-based protons at 0 ppm. The latter implies that hydrolysis occurs over time leading to an increased defect density. Thus, in addition to capillary-force-driven collapse, the process of node-hydrolysis occurs slowly over time even in properly-activated UiO-67. However, given the nominal decrease in SA over the course of a month, the MOF is clearly able to tolerate some hydrolysis of the linker-Zr bonds.
In order to probe the generality of our observations, we repeated the experiment with acetone and dichloromethane as the exchanged solvent (Fig. 4, Fig. S4 and S5, ESI†).17 Samples activated from dichloromethane (Fig. S5, ESI†) showed evidence of amorphous material and was not further examined; we hypothesize that the low miscibility of water with dichloromethane, and thus a less-efficient solvent exchange, is responsible for the degradation of the MOF.
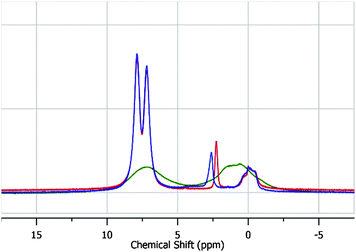 | ||
| Fig. 4 (red) 1H-NMR of UiO-67 immediately after thermal activation from acetone. (blue) 1H-NMR of UiO-67 4 days after thermal activation. (green) 1H-NMR of UiO-67 1 month after thermal activation. The corresponding 13C-NMRs can be seen in Fig. S4 (ESI†). | ||
However, when acetone was utilized (Fig. 4) the SS-NMR of the MOF initially indicated a more stable MOF with respect to hydrolysis (i.e., the peaks at 0 ppm do not shift or increase in intensity). However, after a month, the MOF was found to be completely amorphous with a BET SA of 500 m2 g−1 indicating that, eventually, the MOF succumbs to hydrolysis;18 ultimately, we expect a similar fate to methanol-exchanged UiO-67.
As summarized in Table 1, SS-NMR in combination with SA measurements were used to examine the stability of UiO-67 with respect to time.19 As evident by the changing chemical shift of the μ3-OH and defect-based resonance, SS-NMR is a key tool for the understanding of the dynamic behaviour within MOFs. With respect to the stability of UiO-67, we observed that when solvent molecules remain inside the pore for a few days, then UiO-67 collapses rapidly. However, when solvent is removed at elevated temperatures, then UiO-67 remains stable for at least a month. Inevitably, hydrolysis, caused by the relative humidity, degrades the MOF beyond its structural integrity. In our hands, if kept dry or in solution, UiO-67 remains intact.
| Activation method | Time (days) | BET SA (m2 g−1) | SS-NMR observations |
|---|---|---|---|
| MeOH Wash | 0 | 2000 | MeOH present |
| 6 | 500 | Crystalline and amorphous | |
| Thermal from MeOH | 0 | 2000 | Defects present (0 ppm) |
| 4 | — | μ3-OH shifts downfield, increased defect density | |
| 30 | 1500 | μ3-OH shifts upfield, increased defect density, onset of amorphous material | |
| Thermal from acetone | 0 | 2000 | Defects present (0 ppm) |
| 4 | — | μ3-OH shifts downfield, no change in defect density | |
| 30 | 500 | Completely amorphous | |
| Thermal from DCM (ESI) | 0 | — | Amorphous material observed |
The 600 MHz SS-NMR was funded by CFI. The authors would like to acknowledge NSERC for financial support in the form of a Discovery Grant (MJK) and an Undergraduate Student Research Award (MCL). MJK would also like to acknowledge the RDC for an Ignite grant (MJK).
Notes and references
- (a) J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32–51 RSC; (b) B. Li, H.-M. Wen, W. Zhou and B. Chen, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed; (c) O. K. Farha, A. Ö. Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944–948 CrossRef CAS PubMed; (d) J. B. DeCoste, M. H. Weston, P. E. Fuller, T. M. Tovar, G. W. Peterson, M. D. LeVan and O. K. Farha, Angew. Chem., Int. Ed., 2014, 53, 14092–14095 CrossRef CAS PubMed; (e) Y. Liu, Z. U. Wang and H.-C. Zhou, Greenhouse Gases: Sci. Technol., 2012, 2, 239–259 CrossRef CAS.
- (a) D.-X. Xue, Y. Belmabkhout, O. Shekhah, H. Jiang, K. Adil, A. J. Cairns and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5034–5040 CrossRef CAS PubMed; (b) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed; (c) E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43, 5419–5430 RSC; (d) B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC; (e) S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC.
- (a) C. Y. Lee, O. K. Farha, B. J. Hong, A. A. Sarjeant, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2011, 133, 15858–15861 CrossRef CAS PubMed; (b) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC; (c) S. Jin, H.-J. Son, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 955–958 CrossRef CAS PubMed; (d) K. Meyer, M. Ranocchiari and J. A. van Bokhoven, Energy Environ. Sci., 2015, 8, 1923–1937 RSC.
- (a) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC; (b) G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed; (c) Y. Cui, B. Chen and G. Qian, Coord. Chem. Rev., 2014, 273–274, 76–86 CrossRef CAS.
- (a) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC; (b) C. M. McGuirk, M. J. Katz, C. L. Stern, A. A. Sarjeant, J. T. Hupp, O. K. Farha and C. A. Mirkin, J. Am. Chem. Soc., 2015, 137, 919–925 CrossRef CAS PubMed; (c) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (d) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC; (e) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- (a) M. J. Katz, Z. J. Brown, Y. J. Colon, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC; (b) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed; (c) S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700–7702 RSC; (d) M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS; (e) L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS; (f) M. J. Katz, J. E. Mondloch, R. K. Totten, J. K. Park, S. T. Nguyen, O. K. Farha and J. T. Hupp, Angew. Chem., Int. Ed., 2014, 53, 497–501 CrossRef CAS PubMed; (g) S.-Y. Moon, G. W. Wagner, J. E. Mondloch, G. W. Peterson, J. B. DeCoste, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54, 10829–10833 CrossRef CAS PubMed; (h) M. J. Katz, R. C. Klet, S.-Y. Moon, J. E. Mondloch, J. T. Hupp and O. K. Farha, ACS Catal., 2015, 5, 4637–4642 CrossRef CAS; (i) J. M. Taylor, T. Komatsu, S. Dekura, K. Otsubo, M. Takata and H. Kitagawa, J. Am. Chem. Soc., 2015, 137, 11498–11506 CrossRef CAS PubMed.
- (a) N. Planas, J. E. Mondloch, S. Tussupbayev, J. Borycz, L. Gagliardi, J. T. Hupp, O. K. Farha and C. J. Cramer, J. Phys. Chem. Lett., 2014, 5, 3716–3723 CrossRef CAS PubMed; (b) J. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. DeMarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 10294–10297 CrossRef CAS PubMed.
- (a) D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., 2012, 124, 10453–10456 CrossRef; (b) Z.-Y. Gu, J. Park, A. Raiff, Z. Wei and H.-C. Zhou, ChemCatChem, 2014, 6, 67–75 CrossRef CAS.
- (a) J. Jiang, F. Gándara, Y.-B. Zhang, K. Na, O. M. Yaghi and W. G. Klemperer, J. Am. Chem. Soc., 2014, 136, 12844–12847 CrossRef CAS PubMed; (b) S.-Y. Moon, Y. Liu, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2015, 54, 6795–6799 CrossRef CAS PubMed.
- (a) Y. Huang, W. Qin, Z. Li and Y. Li, Dalton Trans., 2012, 41, 9283–9285 RSC; (b) H. R. Abid, H. M. Ang and S. Wang, Nanoscale, 2012, 4, 3089–3094 RSC.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 CAS.
- J. E. Mondloch, M. J. Katz, N. Planas, D. Semrouni, L. Gagliardi, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 8944–8946 RSC.
- (a) S. Liu, Z. Yue and Y. Liu, Dalton Trans., 2015, 44, 12976–12980 RSC; (b) X. Zhu, B. Li, J. Yang, Y. Li, W. Zhao, J. Shi and J. Gu, ACS Appl. Mater. Interfaces, 2015, 7, 223–231 CrossRef CAS PubMed.
- Caution: desorption of solvent can cause damage to the rotor.
- W. Salomon, C. Roch-Marchal, P. Mialane, P. Rouschmeyer, C. Serre, M. Haouas, F. Taulelle, S. Yang, L. Ruhlmann and A. Dolbecq, Chem. Commun., 2015, 51, 2972–2975 RSC.
- (a) H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed; (b) G. C. Shearer, S. Chavan, J. Ethiraj, J. G. Vitillo, S. Svelle, U. Olsbye, C. Lamberti, S. Bordiga and K. P. Lillerud, Chem. Mater., 2014, 26, 4068–4071 CrossRef CAS; (c) P. Ghosh, Y. J. Colon and R. Q. Snurr, Chem. Commun., 2014, 50, 11329–11331 RSC; (d) M. J. Cliffe, W. Wan, X. Zou, P. A. Chater, A. K. Kleppe, M. G. Tucker, H. Wilhelm, N. P. Funnell, F.-X. Coudert and A. L. Goodwin, Nat. Chem., 2014, 5, 4176 CAS; (e) C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162–11167 CrossRef CAS PubMed.
- J. E. Mondloch, O. Karagiaridi, O. K. Farha and J. T. Hupp, CrystEngComm, 2013, 15, 9258–9264 RSC.
- T. D. Bennett and A. K. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef CAS PubMed.
- Attempts to measure 91Zr-SS-NMR exhibited no decernible signal for UiO-67. NOESY-rfdr experiments showed signals between ligand protons, but no other interactions were observed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 13C-NMR spectra, and 1H-NMR spectra of CH2Cl2-activated UiO-67. See DOI: 10.1039/c5cc09919f |
| This journal is © The Royal Society of Chemistry 2016 |