Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detection of quadrupolar nuclei by ultrafast 2D NMR: exploring the case of deuterated analytes aligned in chiral oriented solvents†
Philippe
Lesot
*ab,
Philippe
Berdagué
a and
Patrick
Giraudeau
*cd
aEquipe RMN en Milieu Orienté, ICMMO, UMR-CNRS 8182, Université de Paris-Sud, Université Paris-Saclay, Rue du Doyen Georges Poitou, Bât. 410, 91405 Orsay cedex, France. E-mail: philippe.lesot@u-psud.fr
bCNRS/INC, 3 rue Michel Ange, 75016 Paris, France
cEquipe EBSI, CEISAM, UMR-CNRS 6230, Université de Nantes, 2, Chemin de la Houssinière, 44300 Nantes, France. E-mail: patrick.giraudeau@univ-nantes.fr
dInstitut Universitaire de France, 1 rue Descartes, 75005 Paris Cedex 05, France
First published on 7th December 2015
Abstract
Anisotropic 2H ultrafast (ADUF) 2D NMR spectroscopy for studying analytes dissolved in chiral liquid crystals (CLC) is investigated for the first time and the analytical possibilities of this method are evaluated. We demonstrate that these unconventional sub-second 2D experiments are compatible with the basic gradient units (40–60 G cm−1) that are implemented in routine spectrometers and allow the recording of 2H signals of weakly aligned deuterated solutes in sub-second experimental times.
A major advantage of proton-decoupled deuterium (2H–{1H}) nD NMR spectroscopy in oriented media such as liquid crystals (LCs) is the access to anisotropic spectral data that are averaged to zero in liquids, such as chemical shift anisotropy (2H CSA) and residual quadrupolar coupling (denoted as RQC(2H) or ΔνQ(2H)). These order-dependent data can provide key information on the molecular geometry, conformational behavior and dynamics of aligned analytes (solutes or mesophases themselves).1
Compared with other nuclei with I > 1/2, deuterons possess a small quadrupolar moment (QD = 2.86 × 10−31 m2),2 which thus limits excessive line broadening owing to quadrupolar relaxation mechanisms. When dissolved in rather weakly oriented LCs such as lyotropic polypeptide systems, they produce 2H quadrupolar doublets (QD), whose magnitude of |ΔνQ| generally ranges from 0 to 1000 Hz. Interestingly, because these aligning media are chiral (helical polymers), the spectral enantiodiscrimination of the enantiotopic directions of deuterated prochiral molecules or enantiomers is possible on the basis of a difference in RQC (ΔνQ(2H)R or pro-R ≠ ΔνQ(2H)S or pro-S).3
Based on this strategy, various methodological developments (2H nD NMR) and a large range of applications (stereochemical analysis) have been proposed, including the detection of deuterium at natural abundance levels (isotope fractionation analysis).4
Very recently, the first monitoring of chiral enzymatic transformations (the interconversion of L- and D-alanine-d3 enantiomers by alanine racemase) directly observed by anisotropic 2H NMR has been successfully pioneered.5 The idea was to follow in situ and in real time the variation in the peak intensity of the QDs of each enantiomer during the bioprocess and determine from the spectral fit the turnover numbers of the enzyme. An inherent drawback of this approach is the experimental time that is required to record spectral data for monitoring the time-dependent variations of all compounds in the mixture, in particular for extremely fast and/or multiple (cascade) chemical transformations in which the fast identification and quantification of 2H signals of products (reactants, (un)stable intermediates, products, etc.) (whether chiral or not) require 2D NMR experiments with sub-minute time resolutions or below.
Non-uniform acquisitions combined with covariance/compressed sensing processing6 have been proposed to reduce the acquisition time in anisotropic NAD 2D NMR experiments. Another appealing option is to rely on ultrafast (UF) 2D NMR, which is an approach capable of yielding homo- or heteronuclear 2D correlations within a single scan when its sensitivity allows this.7 Within the last ten years, the performance of UF experiments has been greatly enhanced by numerous methodological developments, which make UF NMR applicable to a wide range of analytical situations.8 UF NMR has been applied to the real-time monitoring of fast chemical transformations,9 the coupling with other techniques such as chromatography10 or hyperpolarization,11 and also the high-throughput quantitative analysis of complex samples.12
The use of UF 2D NMR experiments in anisotropic environments was described in 201213 for determining residual 13C–1H dipolar couplings (RDC's) from UF heteronuclear spectra. However, such an approach has never been investigated in the case of quadrupolar nuclei such as deuterium (I = 1) using aligned solvents.
In this communication, we report the first examples of anisotropic deuterium UF (ADUF) spectra of deuterated analytes dissolved in lyotropic polypeptide CLCs. We investigate the cases of two isotopically enriched prochiral molecules of Cs symmetry on average, pentanol-d12 (1) and benzyl alcohol (2) enriched in 13C and 2H on the prostereogenic methylene group, both of which possess enantiotopic C–D directions. Although these prochiral molecules possess rather few inequivalent 2H sites (see Fig. 2 and 3), they represent interesting model compounds for discussing the analytical possibilities of ADUF 2D NMR in CLCs and also for investigating the case of spectrally detectable isotopologues.
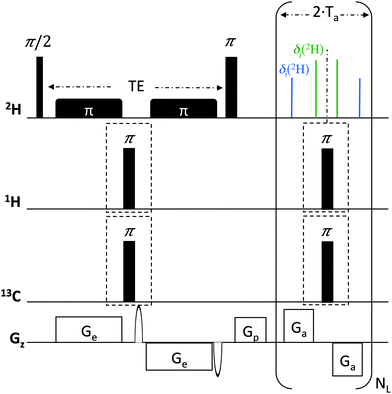
The ADUF 2D pulse sequence shown in Fig. 1 follows the basic scheme of homonuclear UF 2D experiments, where the usual time encoding is replaced by spatial encoding, which is formed by two chirp pulses applied together with a pair of opposite gradients.14 This spatial encoding block is followed by a conventional mixing period and a detection block based on echo planar spectroscopic imaging (EPSI).15 The 2H hard-pulse scheme is identical to the Q-COSY sequence that was developed for conventional 2H experiments (QUOSY-type) and generates magnitude-mode maps.2,3,16 It comprises a 2H 90°(x) pulse followed by a 180°(x) pulse, separated by a spatial encoding period of duration TE. In contrast to conventional 2D Q-COSY experiments, the ADUF 2D scheme is a constant-time experiment, which leads to the refocusing of all coupling patterns in the F2 dimension. Note that in all UF experiments shown here the spatially-encoded dimension is shown in F2, whereas the dimension that results from a Fourier transform of the EPSI domain is shown in F1. In the case of 2H nuclei, the former consists of removing all ΔνQ(2H) in the F2 dimension of the 2D map, together with the possible homonuclear scalar nJ(2H–2H) and dipolar nD(2H–2H) couplings in the case of perdeuterated molecules. In other words, the expected ADUF 2D map is formally identical to that obtained for conventional δ-resolved experiments (QUOSY-type), except that the spectral contents of F1 and F2 are inverted compared with conventional spectra16 (see ESI†). Consequently, ADUF experiments can be considered as 2H δ-resolved constant-time experiments.
 | ||
| Fig. 2 (a) 2H–{1H} 1D spectrum of 1 in a PBLG mesophase. (b) Single-scan ADUF-{1H} 2D map recorded in ca. 400 ms with the pulse sequence of Fig. 1. The X/X′ notation is arbitrarily defined. | ||
Possible nJ(2H–1H) and nD(2H–1H) heteronuclear couplings (which are generally <2 Hz in a PBLG mesophase) can be simply removed by implementing 1H 180° hard pulses between the bipolar gradient pairs, in order to refocus such couplings during the spatial encoding and/or acquisition periods. These pairs of 180° pulses can also be associated with similar pulses on the heteronuclear X channel for further X–2H heteronuclear decoupling. Fig. 1 shows such an optional block for specific decoupling of 13C. Note that owing to the smaller gyromagnetic value of 2H nuclei compared with 1H (γ1H = 6.515 × γ2H), the magnitude of nJ(2H–13C) and nD(2H–13C) couplings remains relatively weak (0 to 50 Hz) and simple 180° pulses are sufficient for efficient decoupling. More complex decoupling schemes might be necessary according to the magnitude of 2H–X couplings, in particular for X nuclei with high values of γ.
Anisotropic samples of 1 (2) were prepared using 100 mg PBLG with a degree of polymerization of 534 (732), 100 (50) mg solute, and 350 mg CHCl3 in a tube of 5 mm o.d. (fire-sealed). Details concerning the preparation of anisotropic samples can be found in references.2,3 The experiments were carried out at 303 K using a Bruker Avance HD spectrometer operating at 16.4 T equipped with an inverse 1H/13C/15N/2H cryogenically cooled probe (107.5 MHz for 2H)17 and including a z-axis gradient.
ADUF 2D spectra were recorded in a single-scan experiment. The following parameters were used for spatial encoding: a bipolar excitation gradient with gradients Ge of 20 ms and 9.7 G cm−1, respectively, and smoothed chirp pulses of 20 ms with a sweep range of 5 kHz. The acquisition gradient parameters were Ta = 716.8 μs and Ga = 58.5 G cm−1. For samples 1 and 2, the number of loops NL was set to 256 and 128, respectively. The 2H π/2 pulse (87 μs/38 W) was carefully calibrated to obtain an accurate 90°/180° excitation. The spatial encoding and acquisition parameters resulted in a spectral width of 1000 Hz, which was sufficient to cover the spectral distribution of 2H information in both dimensions. The 1H and 13C π pulses (21.3 μs/180 W and 22.0 μs/8.5 W, respectively) were also carefully calibrated to ensure optimal refocusing of heteronuclear couplings. Decoupling of 1H can be useful for removing possible residual 1H–2H couplings that are due to incomplete perdeuteration of molecules (in the case of 1) or eliminating any long-range 1H–2H couplings in the case of selectively deuterated solutes (such as 2).
All the ADUF 2D spectra were processed as follows: magnitude mode, zero-filling, Gaussian spatial apodization in the spatially encoded dimension F2,18 and conventional sinebell apodization in F1 to obtain the most symmetrical 2H line shapes and find an optimal compromise between resolution and sensitivity. All the spectra were analyzed using the Bruker program Topspin 3.2. The specific processing of ADUF spectra was performed using a home-written routine in Topspin. This processing program is available on a dedicated website, where a protocol is also provided to help users in the implementation of UF experiments.19 The pulse sequence is available on demand. Other details are given in the figure captions.
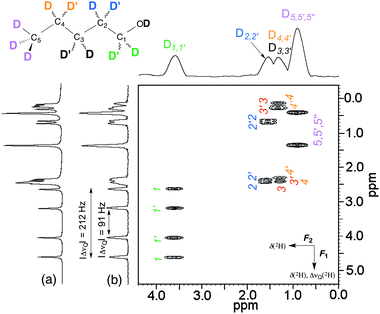
As a first example, we investigated the case of 1-pentanol-d12. This prochiral flexible molecule of Cs symmetry possesses three homotopic C–D directions (from the methyl group) and four pairs of enantiotopic C–D directions, which are associated with the four prostereogenic methylenes of the molecule. A priori, nine QDs are expected to be detected if all inequivalent C–D directions are spectrally discriminated in the CLC, disregarding the hydroxyl group. The ADUF-{1H} 2D spectrum recorded in a single scan is shown in Fig. 2, along with the corresponding projections. Note that all cases of ΔνQ(2H) have been eliminated in F2 and that no tilt is needed because this spatially encoded dimension is constant-time. The F2 dimension is therefore a “pure shift” dimension, which reduces the useful spectral information to δ(2H)aniso only (ca. 500 Hz of width).
This 2D map can be compared with a conventional anisotropic 2H δ-resolved 2D experiment recorded with 128 increments of t1 and eight scans in 17 min (see Fig. S1 and S2 in ESI†). Although the resolutions are lower than that of the conventional 2D map (or 1D spectrum), analysis of the ADUF spectrum shows that the discrimination of enantiotopic site pairs (which is visible for sites 1, 2 and 3) is similar to that observed on the conventional spectrum acquired in 17 min. However, the resolution in the F1 dimension of the ADUF spectrum (Δν1/2(F1) = 5.5 Hz) is limited by the number of loops that the gradient amplifier can support in the EPSI block, whereas this resolution could be improved in the conventional spectrum by increasing the number of increments of t1, although at the expense of time. The resolution in F2 (Δν1/2(F2) = 32 Hz) is a well-known limitation in UF experiments, which is inherent in the need to compromise between resolution, sensitivity and spectral widths.8 Ultimately, the maximum spectral widths in F2 are limited by the maximum amplitude of the acquisition gradients Ga.
Nevertheless, these first results are very promising and clearly indicate that: (i) the detection of 2H nuclei by a UF approach within sub-second experimental times is possible for deuterated solutes; (ii) the gradient power (Gz: 40–60 G cm−1) that is available on gradient units of routine spectrometers is sufficient to yield full-width 2D spectra in a single scan for nuclei with low gyromagnetic ratios – this would not have been possible at high field with 1H owing to the limitations in spectral width of UF NMR; and (iii) the resolution in F1 (a few Hz) allows the discrimination of enantiotopic sites down to differences in |ΔΔνQ|/2 of <6 Hz.
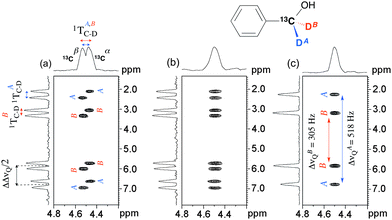
To further examine the spectral possibilities of ADUF 2D experiments, we focused our attention on another solute of Cs symmetry, benzyl alcohol, which was isotopically enriched (2H and 13C) on the methylene group. Owing to the presence of abundant 100% 13C nuclei, four QDs (instead of two for the 2H–12C isotopologue) are observed on the 2H–{1H} 1D spectrum (see Fig. S2, ESI†). The spectral difference between the two pairs of QDs corresponds to the difference in the total couplings of 2H and 13C (|1TA,BC–D| = |1JA,BC–D + 21DA,BC–D|), which is associated with the enantiotopic directions pro-R/pro-S but is simply denoted as “A/B”. From the 1D spectrum several choices for pairing the components are possible, which lead to different values of |1TA,BC–D|.
Fig. 3a presents the single-scan ADUF 2D {2H} map of 2 in the PBLG mesophase that was recorded in 220 ms. In this second experiment, spectral resolutions of ca. 10 Hz (F1) and 32 Hz (F2) were reached; the lower resolution in F1 compared with Fig. 2 is explained by the lower number of EPSI loops. The two resonances observed in F2 are not associated with independent 2H peaks with distinct values of δ(2H) (as for 1) but originate from the total couplings, 1TAC–D and 1TBC–D, the resulting doublet being centered on the value of δ(2H)A,B of the methylene group. In contrast to homonuclear couplings, the evolution of the total heteronuclear 2H–13C couplings is not eliminated by constant-time chemical shift encoding. The presence of this doublet (which is due to the two energy levels of 13C nuclei (|α〉 and |β〉)) and the position of cross-peaks on the 2D map enable the two components of each QD pair (which are associated with the C–DA,B directions) to be easily assigned and then the magnitudes of 1TAC–D and 1TBC–D determined to be equal to +29 and +35 Hz (on the 1D spectrum), respectively. Note that the moderate resolution in F2 leads to one doublet with broad components being detected instead of two resolved doublets.
Interestingly, the data contained on the ADUF 2D spectrum of 2 can be modulated according to the 13C decoupling blocks that were implemented in the TE and Ta periods of the initial pulse sequence (see Fig. 1). On the one hand, the decoupling of 13C during the spatial encoding collapses the doublet into a single resonance that is now centred on δ(2H)pro-R ≈ δ(2H)pro-S (CSA(2H)pro-R ≈ CSA(2H)pro-S) in F2 (see Fig. 3b). On the other hand, the total 13C–2H coupling can also be eliminated by implementing 13C 180° pulses between gradients in the EPSI block, which simplifies the spectral information to two QDs in F1. Applying decoupling of 13C in both dimensions leads to the map shown in Fig. 3c, where the magnitudes of QD can be determined unambiguously (ΔνQ(2H)A = +305 Hz and ΔνQ(2H)B = +518 Hz). The positive sign is consistent with the values of 1TA,BC–D that were measured, considering that 1JA,BC–D = +22 Hz (the ratio “ΔνQ(2H)/1DC–D” = 70–80) for sp3 carbon atoms.
In the present work, we have demonstrated the experimental feasibility and analytical possibilities of anisotropic UF 2D NMR experiments in the case of quadrupolar nuclei such as deuterons. In practice, ADUF experiments are suitable for the study of weakly aligned deuterated solutes because the small magnitudes of RQC(2H) lead to a distribution of resonances over moderate spectral widths (<1000 Hz), which is rather well adapted to the technical limitations of UF experiments. Consequently, these original experiments can be performed on routine NMR spectrometers equipped with basic gradient units when γ1H is 6.5 times less than γ2H. As has been shown, recording all the relevant anisotropic information of perdeuterated analytes is possible within sub-second experiment times, whereas the heteronuclear information (if detectable) can be modulated according to the decoupling scheme that is applied in both dimensions.
These pioneering findings open a vast array of prospects such as: (i) ADUF experiments in which the 2H chemical shifts would be refocused in the EPSI dimension (F1), similarly to Q-resolved 2D experiments;2 (ii) the replacement of a constant-time scheme by a real-time scheme20 to produce maps where the 2H autocorrelations would be distributed perpendicularly to the main diagonal, as in conventional 2H Q-COSY 2D maps; and (iii) the development of algorithms for processing UF spectra in phase-sensitive mode, thus improving the resolution. Although the samples studied in this paper were compatible with the acquisition of full-width spectra in a single scan, the sensitivity and spectral widths may be increased even further, if needed, by relying on hybrid experiments that have recently been described, which combine a few UF scans to improve the analytical performance at a reasonable time cost.21 Such hybrid experiments would also pave the way to the acquisition of fast 3D spectra to reduce peak overlap even further.22
There is no doubt that the ultimate challenge in ADUF experiments is sensitivity. Here, the sensitivity of a single-scan ADUF spectrum was found to be ca. six times lower than that of a single-scan 1D 2H spectrum. This is a well-known limitation of UF NMR.8 Detailed measurements of SNR (see ESI†) show that the acquisition of a single-scan ADUF spectrum with our hardware requires at least 15 mg of 2H-labeled compound in the NMR tube. Although this limit of detection can be lowered by an order of magnitude by signal averaging, the detection of 2H nuclei at natural abundance levels4 will require more sensitive techniques, such as hyperpolarization methods that can be efficiently coupled to UF 2D NMR.11
P. L. and P. B. thank the French CNRS and the Université de Paris-Sud for their recurrent funding of fundamental research.
Notes and references
- (a) P. Lesot, eMagRes, 2013, 2, 315 CAS; (b) C. Aroulanda, O. Lafon and P. Lesot, J. Phys. Chem. B, 2009, 113, 10628 CrossRef CAS PubMed; (c) K. Tabayashi and K. J. Akaska, Liq. Cryst., 1999, 26, 127 CrossRef CAS.
- P. Lesot and J. Courtieu, Prog. Nucl. Magn. Reson. Spectrosc., 2009, 55, 128 CrossRef CAS.
- (a) P. Lesot, C. Aroulanda, H. Zimmerman and Z. Luz, Chem. Soc. Rev., 2015, 44, 2330 RSC; (b) M. Sarfati, P. Lesot, D. Merlet and J. Courtieu, Chem. Commun., 2000, 2069 RSC.
- (a) V. Baillif, R. J. Robins, S. Le Feunten, P. Lesot and I. Billault, J. Biol. Chem., 2009, 284, 10783 CrossRef CAS PubMed; (b) P. Berdagué, P. Lesot, J. Jacob, V.-J. Terwilliger and C. Lemilbeau, Geochim. Cosmochim. Acta, 2016, 173, 337 CrossRef.
- M. Chan-Huot, P. Lesot, P. Pelupessy, L. Duma, G. Bodenhausen, P. Duchambon, M. D. Toney, U. V. Reddy and N. Suryaprakash, Anal. Chem., 2013, 85, 4694 CrossRef CAS PubMed.
- (a) O. Lafon, B. Hu, J.-P. Amoureux and P. Lesot, Chem. – Eur. J., 2011, 17, 6716 CrossRef CAS PubMed; (b) K. Kazimierczuk, O. Lafon and P. Lesot, Analyst, 2014, 139, 2702 RSC.
- L. Frydman, A. Lupulescu and T. Scherf, J. Am. Chem. Soc., 2003, 125, 9204 CrossRef CAS PubMed.
- P. Giraudeau and L. Frydman, Annu. Rev. Anal. Chem., 2014, 7, 129 CrossRef CAS PubMed.
- A. Herrera, E. Fernández-Valle, R. Martínez-Álvarez, D. Molero-Vílchez, Z. D. Pardo-Botero and E. Sáez-Barajas, Magn. Reson. Chem., 2015, 53, 952 CrossRef CAS PubMed.
- L. H. K. Queiroz Jr, D. P. K. Queiroz, L. Dhooghe, A. G. Ferreira and P. Giraudeau, Analyst, 2012, 137, 2357 RSC.
- J. N. Dumez, J. Milani, B. Vuichoud, A. Bornet, J. Lalande-Martin, I. Tea, M. Yon, M. Maucourt, C. Deborde, A. Moing, L. Frydman, G. Bodenhausen, S. Jannin and P. Giraudeau, Analyst, 2015, 140, 5860 RSC.
- T. Jézéquel, C. Deborde, M. Maucourt, V. Zhendre, A. Moing and P. Giraudeau, Metabolomics, 2015, 11, 1231 CrossRef.
- P. Giraudeau, T. Montag, B. Charrier and C. M. Thiele, Magn. Reson. Chem., 2012, 50, S53 CrossRef CAS PubMed.
- P. Pelupessy, J. Am. Chem. Soc., 2003, 125, 12345 CrossRef CAS PubMed.
- P. Mansfield, Magn. Reson. Med., 1987, 1, 370 CrossRef.
- D. Merlet, B. Ancian, J. Courtieu and P. Lesot, J. Am. Chem. Soc., 1999, 121, 5249 CrossRef CAS.
- H. Kovacs, D. Moskau and M. Spraul, Prog. NMR Spectrosc., 2005, 45, 131 CrossRef.
- P. Giraudeau and S. Akoka, Magn. Reson. Chem., 2011, 49, 307 CrossRef CAS PubMed.
- http://www.univ-nantes.fr/giraudeau-p .
- Y. Shrot, B. Shapira and L. Frydman, J. Magn. Reson., 2004, 171, 163 CrossRef CAS PubMed.
- S. Akoka and P. Giraudeau, Magn. Reson. Chem., 2015, 53, 986 CrossRef CAS PubMed.
- (a) R. Boisseau, B. Charrier, S. Massou, J. C. Portais, S. Akoka and P. Giraudeau, Anal. Chem., 2013, 85, 9751 CrossRef CAS PubMed; (b) O. Lafon and P. Lesot, Chem. Phys. Lett., 2005, 404, 90 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Anisotropic 2H 1D and 2D conventional spectra of 1 and 2. See DOI: 10.1039/c5cc09409g |
| This journal is © The Royal Society of Chemistry 2016 |