Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Uncharged nucleoside inhibitors of β-1,4-galactosyltransferase with activity in cells†
Jingqian
Jiang
a,
Varsha
Kanabar
b,
Beatriz
Padilla
b,
Francis
Man
 b,
Simon C.
Pitchford
b,
Clive P.
Page
b and
Gerd K.
Wagner
*a
b,
Simon C.
Pitchford
b,
Clive P.
Page
b and
Gerd K.
Wagner
*a
aDepartment of Chemistry, King's College London, Faculty of Natural & Mathematical Sciences, Britannia House, 7 Trinity Street, London, SE1 1DB, UK. E-mail: gerd.wagner@kcl.ac.uk
bSackler Institute of Pulmonary Pharmacology & Institute of Pharmaceutical Science, King's College London, Faculty of Life Sciences & Medicine, Franklin Wilkins Building, London, SE1 9NH, UK
First published on 16th February 2016
Abstract
We report 5-substituted uridine derivatives as novel, uncharged inhibitors of β-1,4-galactosyltransferase and chemical tools for cellular applications. The new inhibitors reduce P-selectin glycoprotein 1 (PSGL-1) expression in human monocytes. Our results also provide novel insights into a unique mode of glycosyltransferase inhibition.
Glycosyltransferases (GTs) are carbohydrate-active enzymes that catalyze the transfer of a sugar from a glycosyl donor, usually a sugar-nucleotide, to a suitable acceptor, e.g. another sugar, peptide, protein, lipid or small molecule.1–3 The biosynthetic products of GTs are involved in many fundamental biological processes, from cell adhesion4 to bacterial virulence.5 Small molecular GT inhibitors would enable the manipulation of these processes in an operationally simple, reversible, time- and concentration-dependent manner, and are therefore sought after as tool compounds for chemical biology, drug discovery and biotechnology.
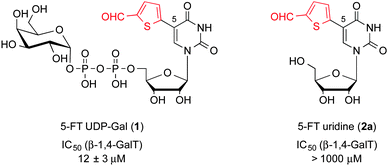
A classical strategy for the development of GT inhibitors is the modification of the natural sugar-nucleotide donor.6 We have recently reported 5-substituted derivatives of the sugar-nucleotide UDP-galactose (UDP-Gal), exemplified by 5-(5-formylthien-2-yl) UDP-Gal 1 (Fig. 1), as a new class of potent galactosyltransferase (GalT) inhibitors.7–9 GalTs are a sub-family of GTs, which catalyse the transfer of D-galactose from UDP-Gal to a specific acceptor.10 Structural and mechanistic studies with a representative blood-group GalT have shown that the 5-substituent in 1 blocks the movement of a flexible loop in the enzyme active site during the catalytic cycle.7,9 Hydrogen bonding and π–π stacking interactions between the 5-(5-formylthien-2-yl) substituent in 1 and the GalT C-terminus and active site loop are essential for this new mode of inhibition.7,9
While 1 is a potent inhibitor of various GalTs in vitro, the presence of the charged pyrophosphate linkage limits its applicability in cell assays. This is a general problem in GT inhibitor discovery, as the pyrophosphate linkage is a key feature of most donor-based GT inhibitors reported to date.6 While there are individual examples for uncharged pyrophosphate mimicry,11 and despite recent progress in this area,12 there is presently no general, uncharged bioisostere for the pyrophosphate group. The complete removal of the pyrophosphate linkage on the other hand usually leads to a dramatic loss of activity. Indeed, no inhibitors based only on the nucleoside part of a given GT donor, i.e. lacking the pyrophosphate group, have been reported to date for any GT. Such uncharged nucleoside inhibitors promise to offer important advantages for practical applications, such as improved stability and cell penetration.
Herein, we describe the first nucleoside inhibitors in the GT family. We have developed derivatives of 1 which contain an optimised 5-substituent, but lack the D-galactose and pyrophosphate moieties. These 5-substituted uridine derivatives act as inhibitors of β-1,4-galactosyltransferase (β-1,4-GalT), but are inactive against a related α-1,4-GalT which uses the same UDP-Gal donor. Our results provide important insights into the structural factors that determine activity and target selectivity in this new GT inhibitor class. We also show that a representative inhibitor in this new series reduces the expression of the cell surface glycoprotein P-selectin glycoprotein ligand-1 (PSGL-1) in human monocytes. The new inhibitors will therefore be valuable as novel tool compounds for cellular applications.
In an initial attempt to obtain an uncharged inhibitor of β-1,4-GalT, we formally removed the pyrophosphate and D-galactose moieties in 1. However, while 1 inhibits β-1,4-GalT with an IC50 value of 12 μM, the corresponding uridine derivative 2a is inactive at concentrations up to 1 mM (Fig. 1). In order to overcome the dramatic drop in activity resulting from removal of the pyrophosphate linkage and D-galactose, we sought to optimise the 5-substituent in 2a. We therefore prepared a series of uridine derivatives with different aromatic and heteroaromatic substituents in position 5 (Scheme 1, 2b–j). The target molecules were obtained by Suzuki–Miyaura coupling of 5-iodouridine with different aryl and heteroaryl boronic acids (Scheme 1). We7–9 and others13,14 have previously reported conditions for the cross-coupling of unprotected uridine nucleosides and nucleotides in aqueous media. It is well known that electron-deficient (hetero)aryl boronic acids tend to react sluggishly in cross-coupling reactions under conventional heating.15 In order to facilitate the cross-coupling of such less reactive boronic acids, we explored suitable microwave conditions for the Suzuki–Miyaura reaction of 5-iodouridine. For 5-formylthien-2-yl boronic acid, microwave conditions led to markedly improved yields, from 26% to 54%, and significantly reduced reaction times, from 2 days to 0.5 h, compared to conventional heating (Table S1, entries 1 and 2, ESI†). These general conditions, employed with several slightly different catalytic systems, allowed the generation of a family of 5-substituted uridines (Table S1, ESI†). Although yields were variable, this method allowed the rapid generation of each uridine derivative in sufficient quantities for biological testing.
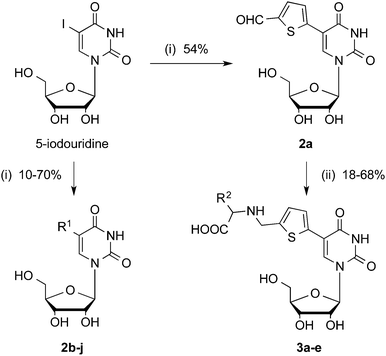 | ||
| Scheme 1 Synthesis of uridine derivatives 2 and 3. Reagents and conditions: (i) boronic acid, Pd catalyst, ligand, base, solvents, microwave irradiation; (ii) amino acids, NaBH3CN, MeOH, rt, 1 d. For details and individual yields see ESI,† for R1/R2 see Table S3 (ESI†). | ||
In order to generate additional structural diversity, we next subjected 5-(5-formylthien-2-yl) uridine 2a to reductive amination with several amino acids16 (Scheme 1, 3a–e). We speculated that through extension of the 5-substituent, these derivatives might be able to pick up additional interactions with the target enzyme β-1,4-GalT. For these modifications, amino acids with different side chain functionalities were chosen, including glutamic acid, lysine, tryptophan and valine. The isolated yields of the reductive amination products were generally modest, ranging from 18–68% (Table S2, ESI†).
In order to assess the inhibitory activity of our collection of 5-substituted uridine derivatives towards β-1,4-GalT, we used a biochemical assay, which we have recently adapted for inhibitor experiments.17 In this colorimetric assay, the phosphate groups of the secondary GT reaction product UDP are cleaved by a phosphatase, and the resulting inorganic phosphate is quantified with malachite green.17 To quantify inhibitory activity of candidate molecules, we determined IC50 values at enzyme turnover rates between 20–50% of UDP-Gal donor (ESI†). We have previously established that this turnover window gives reproducible and comparable results.17
In the first round of screening, uridine derivatives 2a–g and 3a–e were tested against β-1,4-GalT under these conditions (Table S3, ESI†). While most of these derivatives were inactive at concentrations up to 1 mM, 2e and 3e showed promising inhibitory activity (Table 1). Intriguingly, the 5-substituent of both of these derivatives contains an indole motif. We therefore synthesised another three uridine derivatives specifically with an indole substituent in position 5 (Table S3, ESI†, 2h–j). Encouragingly, two of these derivatives showed comparable or better activity than 2e and 3e, suggesting that an indole substituent in position 5 is indeed advantageous for inhibitory activity in this series.
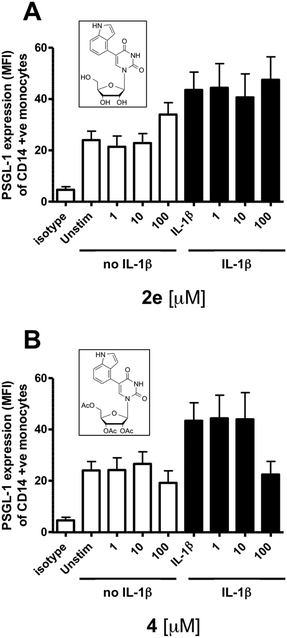
Next, we wanted to assess if these new β-1,4-GalT inhibitors may also be active in cells. In humans, β-1,4-GalT is required for the formation of the glycan epitope Sialyl Lewis X (sLex) on PSGL-1, a key adhesion molecule involved in inflammatory cell recruitment into tissues.18 The expression of PSGL-1 in human monocytes is upregulated upon treatment of cells with a pro-inflammatory stimulus such as IL-1β. Using an established FACS-based assay (ESI†),19 we tested the effect of the representative inhibitor 2e on basal (no IL-1β-stimulation) and IL-1β-induced PSGL-1 expression in human monocytes.
Under either of these conditions, 2e showed no effect on PSGL-1 expression (Fig. 2A). We reasoned that this lack of activity may be due to the limited cell uptake of this uncharged, but still relatively polar nucleoside. Taking advantage of the absence of the synthetically problematic D-galactose and pyrophosphate moieties, we converted 2e into its fully acetylated pro-drug 4 in a single synthetic step (Scheme S1, ESI†). The use of peracetylated sugars and nucleosides is a well established pro-drug strategy, as the acetyl groups are readily removed by intracellular carboxylesterases.20
Pleasingly, at a concentration of 100 μM, 4 reduced excess PSGL-1 expression in IL-1β-stimulated hPBMCs to basal levels, but did not affect PSGL-1 expression in unstimulated hPBMCs (Fig. 2B). This pronounced and selective inhibition of PSGL-1 expression in cells is consistent with the β-1,4-GalT inhibitory activity of 2ein vitro. These results therefore suggest that pro-drug 4 is readily taken up into cells and converted intracellularly into its parent compound 2e, which very likely is the actual active species. Importantly, at the concentrations used in the PSGL-1 expression assay, neither 2e nor 4 affected the cell viability of hPBMCs (ESI†).
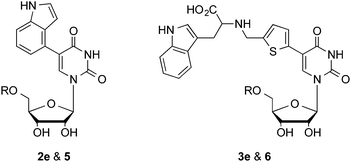
5-FT UDP-Gal 1 is a broad-spectrum GalT inhibitor with activity against several different GalTs.7,8 In order to gain insight into the target selectivity of the new nucleoside-based inhibitors, we also tested 2e and 3e against the α-1,4-GalT LgtC from Neisseria meningitidis,21 which uses the same UDP-Gal donor substrate as β-1,4-GalT. Both 2e and 3e were inactive against LgtC at the highest concentration tested (Table 1). This result suggests that removal of the sugar diphosphate motif does not only represent a strategy to identify inhibitors with better access to cells, but also offers an opportunity to achieve target selectivity between different GalTs.
In order to better understand the respective contribution of the 5-substituent and the pyrophosphate and D-galactose groups to GalT inhibition, we also prepared the UDP-Gal derivatives corresponding to 2e and 3e. These new UDP-Gal derivatives 5 and 6 (Scheme S2, ESI†) were obtained using a modification of our previous synthetic route towards such 5-substituted UDP-sugars.7,8 Thus, the 5-substituent was introduced by cross-coupling of 5-iodo UMP phosphoromorpholidate, followed by pyrophosphate bond formation with galactose-1-phosphate (Scheme S2, ESI†).
As anticipated, the addition of the pyrophosphate and D-galactose groups improved inhibitory activity against β-1,4-GalT (Table 1). Thus, UDP-sugars 5 and 6 were 5-fold more active than the corresponding nucleosides 2e and 3e. Intriguingly, however, the rank order within the UDP-sugar series was not as expected. UDP-sugars 5 and 6 were slightly weaker inhibitors of β-1,4-GalT than the prototype inhibitor 5-(5-formylthien-2-yl) UDP-Gal 1, even though the corresponding nucleosides 2e and 3e are superior to 5-(5-formylthien-2-yl) uridine 2a (Table 1 and Fig. 1). Equally interestingly, in contrast to the corresponding nucleosides, the UDP-Gal derivatives showed no selectivity for β-1,4-GalT, and were also active against α-1,4-GalT, with very similar potency (Table 1).
These results suggest that the contribution to β-1,4-GalT inhibition of the 5-substituent on the one hand, and the pyrophosphate and D-galactose groups on the other, is not additive. Rather, there appears to be a direct interplay for GalT inhibition between these different moieties. In the presence of the complete UDP-Gal structure, the pyrophosphate group appears to be the dominant factor for inhibition, which enables strong inhibition, even if the nature of the 5-substituent is sub-optimal (cf.1vs.5 and 6). On the other hand, this generic binding motif also leads to a loss of selectivity, as evidenced by the indiscriminate activity of 5 and 6 against both GalTs tested. In contrast, in the absence of the pyrophosphate and D-galactose groups, the nature of the 5-substituent becomes critical not only for this mode of inhibition in general, but also for inhibitory potency. In the case of β-1,4-GalT, indole-based 5-substituents appear to be favourable, probably due to specific interactions with this target enzyme. For other GalTs, the nature of these interactions may well be different, due to the different architecture of the flexible loop and C-terminal region in different enzymes. The 5-substituted uridine inhibitors therefore provide an opportunity for the design of target-selective GalT inhibitors.
We have developed the first nucleoside-based inhibitors of glycosyltransferases. In contrast to previously reported donor-based GT inhibitors, these nucleosides are uncharged, which facilitates their application in cell assays. Proof-of-concept experiments demonstrate that these new inhibitors can indeed be used to reduce the expression of the cell surface glycoprotein PSGL-1 in human monocytes. The new inhibitors will therefore be useful tool compounds for chemical biology and biotechnology, as well as starting points for drug discovery programmes searching for novel anti-inflammatory agents. Due to their fragment-like nature, the new inhibitors can be further optimised using approaches such as fragment-based design22 and dynamic combinatorial chemistry. Efforts in this direction are currently underway.
We are grateful to the volunteers who donated blood samples for this study. We thank King's College London for a PGR studentship (to JJ), the EPSRC National Mass Spectrometry Facility, Swansea, for the recording of mass spectra, and Dr Niina Goos for the expression and purification of LgtC. Plasmids were generous gifts from Dr Christelle Breton, Grenoble (β-1,4-GalT) and Dr Warren Wakarchuk, Toronto (LgtC).
Notes and references
- T. M. Gloster, Advances in understanding glycosyltransferases from a structural perspective, Curr. Opin. Struct. Biol., 2014, 28, 131–141 CrossRef CAS PubMed.
- L. L. Kiessling and R. A. Splain, Chemical approaches to glycobiology, Annu. Rev. Biochem., 2010, 79, 619–653 CrossRef CAS PubMed.
- L. L. Lairson, B. Henrissat, G. J. Davies and S. G. Withers, Glycosyltransferases: Structures, functions, and mechanisms, Annu. Rev. Biochem., 2008, 77, 521–555 CrossRef CAS PubMed.
- J. D. Marth and P. K. Grewal, Mammalian glycosylation in immunity, Nat. Rev. Immunol., 2008, 8, 874–887 CrossRef CAS PubMed.
- H. L. P. Tytgat and S. Lebeer, The Sweet Tooth of Bacteria: Common Themes in Bacterial Glycoconjugates, Microbiol. Mol. Biol. Rev., 2014, 78, 372–417 CrossRef CAS PubMed.
- S. Wang and S. Vidal, Recent design of glycosyltransferase inhibitors, Carbohydr. Chem., 2013, 39, 78–101 CrossRef PubMed.
- T. Pesnot, R. Jørgensen, M. M. Palcic and G. K. Wagner, Structural and mechanistic basis for a new mode of glycosyltransferase inhibition, Nat. Chem. Biol., 2010, 6, 321–323 CrossRef CAS PubMed.
- K. Descroix, T. Pesnot, Y. Yoshimura, S. S. Gehrke, W. Wakarchuk, M. M. Palcic and G. K. Wagner, Inhibition of galactosyltransferases by a novel class of donor analogues, J. Med. Chem., 2012, 55, 2015–2024 CrossRef CAS PubMed.
- R. Jørgensen, T. Pesnot, H. J. Lee, M. M. Palcic and G. K. Wagner, Base-modified donor analogues reveal novel dynamic features of a glycosyltransferase, J. Biol. Chem., 2013, 288, 26201–26208 CrossRef PubMed.
- T. Hennet, The galactosyltransferase family, Cell. Mol. Life Sci., 2002, 59, 1081–1095 CrossRef CAS PubMed.
- T. S. Elliott, A. Slowey, Y. L. Ye and S. J. Conway, The use of phosphate bioisosteres in medicinal chemistry and chemical biology, MedChemComm, 2012, 3, 735–751 RSC.
- S. A. Wang, J. A. Cuesta-Seijo, D. Lafont, M. M. Palcic and S. Vidal, Design of Glycosyltransferase Inhibitors: Pyridine as a Pyrophosphate Surrogate, Chem. – Eur. J., 2013, 19, 15346–15357 CrossRef CAS PubMed.
- K. H. Shaughnessy, Palladium-catalyzed modification of unprotected nucleosides, nucleotides and oligonucleotides, Molecules, 2015, 20, 9419–9454 CrossRef CAS PubMed.
- G. Herve, G. Sartori, G. Enderlin, G. Mackenzie and C. Len, Palladium-catalyzed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides, RSC Adv., 2014, 4, 18558–18594 RSC.
- A. J. J. Lennox and G. C. Lloyd-Jones, Selection of boron reagents for Suzuki–Miyaura coupling, Chem. Soc. Rev., 2014, 43, 412–443 RSC.
- Y. Kabri, M. D. Crozet, R. Szabo and P. Vanelle, Efficient and Original Microwave-Assisted Suzuki–Miyaura Cross-Coupling Reaction in the 4H-Pyrido[1,2-a]pyrimidin-4-one Series, Synthesis, 2011, 3115–3122 CAS.
- L. Tedaldi, A. Evitt, N. Göös, J. Jiang and G. K. Wagner, A practical glycosyltransferase assay for the identification of new inhibitor chemotypes, MedChemComm, 2014, 5, 1193–1201 RSC.
- M. Sperandio, Selectins and glycosyltransferases in leukocyte rolling in vivo, FEBS J., 2006, 273, 4377–4389 CrossRef CAS PubMed.
- V. Kanabar, L. Tedaldi, X. Nie, I. Panina, K. Descroix, F. Man, S. C. Pitchford, C. P. Page and G. K. Wagner, manuscript submitted.
- J. R. Brown, F. Yang, A. Sinha, B. Ramakrishnan, Y. Tor, P. K. Qasba and J. D. Esko, Deoxygenated Disaccharide Analogs as Specific Inhibitors of β-1,4-Galactosyltransferase 1 and Selectin-mediated Tumor Metastasis, J. Biol. Chem., 2009, 284, 4952–4959 CrossRef CAS PubMed.
- K. Persson, H. D. Ly, M. Dieckelmann, W. W. Wakarchuk, S. G. Withers and N. C. J. Strynadka, Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs, Nat. Struct. Biol., 2001, 8, 166–175 CrossRef CAS PubMed.
- D. E. Scott, A. G. Coyne, S. A. Hudson and C. Abell, Fragment-Based Approaches in Drug Discovery and Chemical Biology, Biochemistry, 2012, 51, 4990–5003 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc09289b |
| This journal is © The Royal Society of Chemistry 2016 |