Open Access Article
Open Access ArticleSwitching first contact: photocontrol of E. coli adhesion to human cells†
L.
Möckl‡
a,
A.
Müller‡
b,
C.
Bräuchle
*a and
T. K.
Lindhorst
*b
aDepartment of Physical Chemistry, Ludwig Maximilian University of Munich, Butenandtstr. 11, D-81377 Munich, Germany
bOtto Diels Institute of Organic Chemistry, Christiana Albertina University of Kiel, Otto-Hahn-Platz 3/4, D-24118 Kiel, Germany. E-mail: tklind@oc.uni-kiel.de; Fax: +49 431-8807410
First published on 19th November 2015
Abstract
We have shown previously that carbohydrate-specific bacterial adhesion to a non-physiological surface can be photocontrolled by reversible E/Z isomerisation using azobenzene-functionalised sugars. Here, this approach is applied to the surface of human cells. We show not only that bacterial adhesion to the azobenzene glycoside-modified cell surface is higher in the E than in the Z state, but add data about the specific modulation of the effect.
Cell adhesion is a fundamental principle of biology. Many of its aspects have been elucidated,1,2 but the molecular details of cell interactions are not fully understood. As the first contact of cells is mediated through their glycosylated surfaces, our research on cell adhesion is focused on carbohydrate recognition.
All organisms can recognise and distinguish between carbohydrates with the help of specialised proteins, the lectins.3 Lectin function has been described in the context of, i.e., signalling, trafficking, and quality control, thus involving indispensable processes of life.4,5 Also bacterial cells utilise their own lectins to mediate adhesion to glycosylated surfaces such as the membrane of the target cells.6,7 Furthermore, bacterial adhesion enables bacterial colonization, biofilm formation, biofouling, or it precedes infection of cells.8,9 Hence, investigation and control of bacterial adhesion is important, especially in a medical context, given the high incidence rates of infectious diseases worldwide.10
In recent years we have studied the α-D-mannoside-specific adhesion of Escherichia coli bacteria,11,12 which is mediated by the bacterial lectin FimH. FimH is located at the tips of adhesive organelles, called type 1 fimbriae,13 which are projecting from the bacterial cell surface. Previously we have shown that type 1 fimbriae-mediated bacterial adhesion to a non-physiological surface can be photochemically controlled. In this specific case azobenzene-functionalised α-D-mannoside derivatives were assembled on a gold surface in the form of a glyco-SAM (self-assembled monolayer).14,15 Photochemical isomerisation of the azobenzene moiety between its E and Z form then allowed to reversibly switch the orientation of the attached carbohydrate ligands. In parallel with this E/Z isomerisation, the adhesiveness of the surface was altered leading to reduction of bacterial adhesion in the Z state of the SAM by ∼80% in comparison to the E state.
While the azobenzene photoswitch is long known16 and has been used before for switching of the properties of designer surfaces,17–20 our work on the control of bacterial adhesion through re-orientation of carbohydrate presentation is unprecedented. Here it has been our goal to challenge our system in the context of cell–cell adhesion, thus changing the artificial glyco-SAM surface against the plasma membrane of human cells. For this, we had to incorporate azobenzene mannosides in the cell surface of live cells, effect their E/Z isomerisation to switch the orientation of the attached mannoside moieties, and to finally test the influence of this isomerisation on E. coli adhesion.
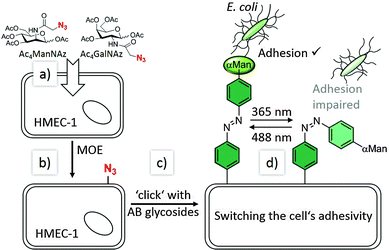
Our experimental approach is explained in Fig. 1: metabolic oligosaccharide engineering (MOE)21,22 was employed to install bioorthogonal azido groups on the cell surface. This allows their subsequent modification with alkyne-functionalised azobenzene glycosides by well-known click chemistry.23–26 In a final step, photochemical E/Z isomerisation of the installed azobenzene moieties was effected by irradiation with light of the appropriate wavelength, and subsequently bacterial adhesion was measured via high-resolution live-cell fluorescence microscopy. For these experiments, human microvascular endothelial cells, variant 1 (HMEC-1) and GFP-fluorescent type 1 fimbriated E. coli PKL116227 were used.
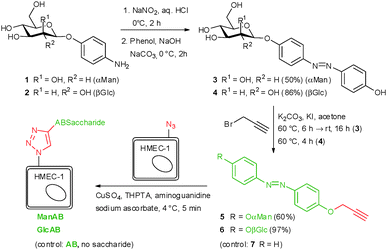
To install the required bioorthogonal azido functional groups on the cell surface, HMEC-1 were incubated with 50 μM tetraacetylated N-azidoacetyl-D-mannosamine (Ac4ManNAz, Fig. 1). Ac4ManNAz is taken up by the cells and proceeded to result in azido labelling of terminal sialic acid units of membrane glycoproteins. When Ac4GalNAz is employed on the other hand, the azido label is incorporated into mucin-type glycoproteins.28 For the chemical functionalisation of the azido-modified cells, the azobenzene derivatives 5–7, carrying an alkyne functional group were required (Scheme 1). Synthesis started with the known p-aminophenyl glycosides 127 and 2,29 which were subjected to a classical azo coupling with phenol to furnish the respective hydroxyazobenzene derivatives 3 and 4.30 Then, Williamson etherification with propargyl bromide led to the desired alkyne-functionalised azobenzene glycosides 5 and 6. The azobenzene alkyne 7 was needed as control and was directly obtained from hydroxyazobenzene.31
At first, the azobenzene α-D-mannoside 5 was conjugated to azido-functionalised HMEC-1 after Ac4ManNAz labelling. For this reaction, 200 μM azobenzene α-D-mannoside, 50 μM CuSO4, 250 μM tris(3-hydroxypropyltriazolylmethyl)amine, 1 mM aminoguanidine, and 2.5 mM sodium ascorbate in buffered saline solution were employed at 4 °C for 5 minutes.32 A 200 μM solution of 5 was sufficient to ensure complete labelling of all azido groups on the cell surface (cf. ESI,† Fig. S13). Copper(I), which is produced during the reaction, is known to exhibit toxic side effects after some time, however, we applied conditions that were shown earlier not to be harmful for cells.
Labelled cells were split into two portions and both sets were irradiated with UV light (365 nm), to effect E → Z isomerisation of the conjugated azobenzene moiety. Then, cells were incubated with GFP-fluorescent E. coli (cf. ESI,† Fig. S14–S18) and the number of adhered bacteria was counted employing high-resolution live-cell fluorescence microscopy. For both sets of HMEC-1 the number of adhered bacteria is similarly low at this stage, as it is expected (Fig. 2).
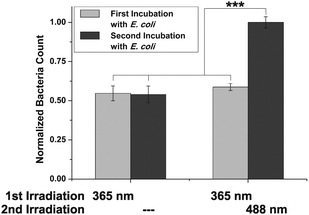 | ||
| Fig. 2 Switching of ManAB configuration allows to control adhesion of E. coli. After incubation of two sets of Ac4ManNAz-engineered HMEC-1, conjugated with 5 in Z-configuration, bacterial adhesion is similar for both sets. Switching of ManAB configuration to E (365 nm) increases bacterial adhesion (second incubation). Error bars are standard error of the mean (SEM) of experiments with four independent sets of cells/condition. ***p < 0.001 (cf. ESI,† Fig. S14). | ||
In a subsequent step, only one of both cell sets was irradiated again; this time with green light (488 nm) to reverse the initial isomerisation and thus effect Z → E isomerisation of the azobenzene configuration. Then, a second incubation with fluorescent E. coli was performed, and adhesion again quantified. Whereas the adhesivity of the cells without a second irradiation treatment remained the same, a significant increase of bacterial adhesion was measured for the cells that were irradiated at 488 nm (about 50%). We attribute this observation to the mode of mannose orientation, which is changed with the isomerisation of the azobenzene hinge. In accordance with the observation made with photoswitchable glyco-SAMs,15 the adhesivity of the azobenzene mannoside-modified cell surface is higher in the E than in the Z state.
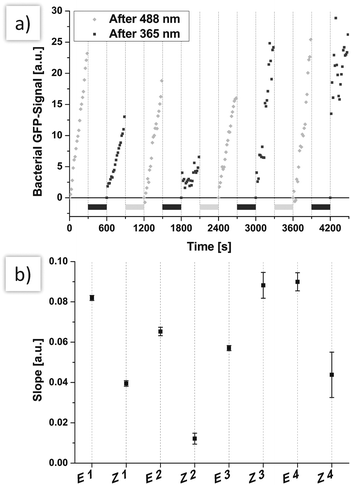
In order to investigate photoswitching of cell adhesion in real-time, flow-based experiments33 were performed next. Thus, HMEC-1 were cultivated in flow chambers, again incubated with Ac4ManNAz and labelled with azobenzene mannoside 5 in analogy to our experiments under static conditions. Then, a bacterial suspension (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dilution of a suspension with OD600 = 0.1) was employed at continuous flow with a shear rate of 1.5 dyn cm−2 (a typical shear rate for microvascules). The flow was not interrupted during irradiation (300 seconds). Adhering bacteria were monitored by fluorescence microscopy while switching the configuration of HMEC-conjugated azobenzene mannosides multiple times between E and Z (Fig. 3). The development of the recorded bacterial GFP-fluorescence is then a direct measure for the adhesion of E. coli. The obtained curves clearly show that alternating irradiation using light of 365 nm and 488 nm has a strong effect on bacterial adhesion (Fig. 3a). After irradiation of the flow chamber with 488 nm light, the slopes of the bacterial GFP signal are significantly higher than after irradiation with 365 nm. This is also clearly seen after fitting of the curves, assuming a linear development of fluorescence (Fig. 3b).
50 dilution of a suspension with OD600 = 0.1) was employed at continuous flow with a shear rate of 1.5 dyn cm−2 (a typical shear rate for microvascules). The flow was not interrupted during irradiation (300 seconds). Adhering bacteria were monitored by fluorescence microscopy while switching the configuration of HMEC-conjugated azobenzene mannosides multiple times between E and Z (Fig. 3). The development of the recorded bacterial GFP-fluorescence is then a direct measure for the adhesion of E. coli. The obtained curves clearly show that alternating irradiation using light of 365 nm and 488 nm has a strong effect on bacterial adhesion (Fig. 3a). After irradiation of the flow chamber with 488 nm light, the slopes of the bacterial GFP signal are significantly higher than after irradiation with 365 nm. This is also clearly seen after fitting of the curves, assuming a linear development of fluorescence (Fig. 3b).
Only after several switching events (starting at 1500 s), the measured slopes are no longer correlated with azobenzene configuration. This can be attributed to increasing coverage of HMEC-1 with bacterial cells (see also microscopic images, ESI,† Fig. S19). When more and more bacteria adhere to bacterial cells and not to HMEC-1, the adhesion is naturally not sensitive to E/Z isomerisation.
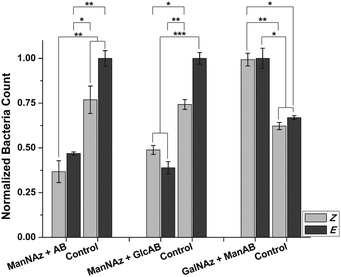
As under static conditions, the results obtained in the flow experiments indicate that the orientation of mannoside 5 conjugated to the surface of HMEC-1 has a strong influence on the adhesion of bacteria, suggesting that the bent Z form is less accessible for bacteria than the E form. Thus, adhesion is reduced after E → Z isomerisation. Next, two important control experiments were performed in order to understand the observed effects in greater detail. First, the question of carbohydrate specificity of the observed effects was addressed by employing the azobenzene derivative 7 (AB in Fig. 4) devoid of a carbohydrate, and the β-D-glucoside 6 (GlcAB in Fig. 4) for HMEC-1 labelling. The glucoside 6 exhibits a similar structure and polarity as 5 but is no ligand for the bacterial lectin FimH. Labelling of HMEC-1 was done as before and bacterial adhesion measured after irradiation. All experiments were compared to azido-labelled HMEC-1, which had not been reacted with either of the azobenzene derivatives (Control in Fig. 4).
These experiments show that adhesion of E. coli to cells reacted with either 6 or 7, is significantly decreased compared to untreated cells. This observation suggests a shielding effect for both derivatives, prohibiting bacterial adhesion to the surface of HMEC-1 to some extent. On the other hand, this effect is not significantly sensitive to E/Z isomerisation. This finding indicates that the adhesion of bacteria to cells treated with 5 is indeed carbohydrate-specific. Control cells showed a slight response to irradiation with 365 nm light, but the effects caused by 6 or 7 are more significant.
How can the orientation of a rather small molecule (such as an azobenzene glycoside) within the complex environment of a cell's surface exert such a pronounced effect on cell adhesion? To answer this question, the azobenzene mannoside ligand was positioned differently on the surface of HMEC-1. This is possible by labelling HMEC-1 with Ac4GalNAz instead of Ac4ManNAz. Whereas Ac4ManNAz leads to labelling of sialic acids, which are almost exclusively found at the terminal positions of cell surface glycans, Ac4GalNAz labels mucin-type glycoproteins. These are characterised by LacNAc moieties, which are localised deeper within the glycosylated cell surface.28
Indeed, bacterial adhesion to HMEC-1, labelled with Ac4GalNAz and modified with mannoside 5 is increased in comparison to control cells (Fig. 4). This observation can be rationalised by the increased concentration of mannosides present on the cell surface after modification. However, the observed increased adhesion is not photosensitive in this case. As LacNAc groups are typically not exposed at the cell surface, conjugated ManAB moieties might be buried under a layer of other glycoconjugates so that an orientational change of mannoside ligands is not effective. On the other hand, we cannot measure the extent of azobenzene isomerisation, which might be different in both investigated cases (Ac4ManNAz or Ac4GalNAz labelling).
Taken together, we show that the azobenzene mannoside 5 can be employed to influence adhesion of E. coli to HMEC-1. We postulate the following mechanism that underlies this photocontrol: (i) bacterial FimH binds to mannose, which serves as ligand for specific interaction between the bacterium and the modified cell surface (cf. Ac4ManNAz + 5vs. Ac4ManNAz + 6). (ii) If no mannose ligand is present or if it adopts a disadvantageous orientation (Z-configuration), the azobenzene groups shield the cell, leading to reduced binding of E. coli (cf. Ac4ManNAz + 5vs. Ac4ManNAz + AB). (iii) The FimH ligand mannose must be localised at the terminus of glycans to ensure that the change in configuration upon a Z/E transition is not small (Ac4ManNAz + 5vs. Ac4GalNAz + 5).
It is surprising that this orientation effect can be found not only on an artificial, ordered glyco-SAM as we described before,15 but even in the complex, in comparison rather chaotic setting of the cell surface. Of course, this finding bears implications for the way how cells interact with their environment and offers options for prevention of bacterial adhesion with temporal and spatial resolution.
Financial support by SFB 677 is gratefully acknowledged. We also thank the excellence clusters Nano Systems Initiative Munich (NIM), the Centre for Integrated Protein Science Munich (CIPSM), and the Centre for Nanoscience Munich (CeNS).
Notes and references
- A. A. Khalili and M. R. Ahmad, Int. J. Mol. Sci., 2015, 16, 18149–18184 CrossRef PubMed.
- M. E. Taylor and K. Drickamer, Curr. Opin. Cell Biol., 2007, 19, 572–5772 CrossRef CAS PubMed.
- N. Sharon and H. Lis, Glycobiology, 2004, 14, 53R–62R CrossRef CAS PubMed.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed.
- F.-T. Liu and G. A. Rabinovich, Nat. Rev., 2005, 5, 29–41 CAS.
- I. Ofek, D. Mirelman and N. Sharon, Nature, 1977, 265, 623–625 CrossRef CAS PubMed.
- K. Ohlsen, T. A. Oelschlaeger, J. Hacker and A. S. Khan, Top. Curr. Chem., 2009, 288, 17–65 CrossRef CAS PubMed.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- D. Ribet and P. Cossart, Microbes Infect., 2015, 17, 173–183 CrossRef CAS PubMed.
- K. E. Jones, N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman and P. Daszak, Nature, 2008, 451, 990–993 CrossRef CAS PubMed.
- M. Hartmann and T. K. Lindhorst, Eur. J. Org. Chem., 2011, 3583–3609 CrossRef CAS.
- A. Bernardi, et al. , Chem. Soc. Rev., 2013, 42, 4709–4727 RSC.
- J. Lillington, S. Geibel and G. Waksman, Biochim. Biophys. Acta, 2014, 1840, 2783–2793 CrossRef CAS PubMed.
- V. Chandrasekaran, H. Jacob, F. Petersen, K. Kathirvel, F. Tuczek and T. K. Lindhorst, Chem. – Eur. J., 2014, 20, 8744–8752 CrossRef CAS PubMed.
- T. Weber, V. Chandrasekaran, I. Stamer, M. B. Thygesen, A. Terfort and T. K. Lindhorst, Angew. Chem., 2014, 126, 14812–14815 ( Angew. Chem., Int. Ed. , 2014 , 53 , 14583–14586 ) CrossRef.
- G. S. Hartley, Nature, 1937, 140, 281 CrossRef CAS.
- J. Auernheimer, C. Dahmen, U. Hersel, A. Bausch and H. Kessler, J. Am. Chem. Soc., 2005, 127, 16107–16110 CrossRef CAS PubMed.
- P. M. Mendes, Chem. Soc. Rev., 2008, 37, 2512–2529 RSC.
- W. R. Browne and B. L. Feringa, Annu. Rev. Phys. Chem., 2009, 60, 407–428 CrossRef CAS PubMed.
- M.-M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666–676 CrossRef CAS PubMed.
- M. S. Siegrist, B. M. Swarts, D. M. Fox, S. A. Lim and C. R. Bertozzi, FEMS Microbiol. Rev., 2015, 39, 184–202 CrossRef PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., 2002, 114, 2708–2711 ( Angew. Chem., Int. Ed. , 2002 , 41 , 2596–2599 ) CrossRef.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- M. Kleinert, T. Winkler, A. Terfort and T. K. Lindhorst, Org. Biomol. Chem., 2008, 6, 2118–2132 CAS.
- C. Grabosch, M. Kind, Y. Gies, F. Schweighöfer, A. Terfort and T. K. Lindhorst, Org. Biomol. Chem., 2013, 11, 4006–4015 CAS.
- M. Hartmann, A. K. Horst, P. Klemm and T. K. Lindhorst, Chem. Commun., 2010, 46, 330–332 RSC.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- G. R. Gustafson, C. M. Baldino, M.-M. E. O'Donnell, A. Sheldon, R. J. Tarsa, C. J. Verni and D. L. Coffen, Tetrahedron, 1998, 54, 4051–4065 CrossRef CAS.
- V. Chandrasekaran, E. Johannes, H. Kobarg, F. D. Sönnichsen and T. K. Lindhorst, ChemistryOpen, 2014, 3, 99–108 CrossRef CAS PubMed.
- G. Mantovani, V. Ladmiral, L. Tao and D. M. Haddleton, Chem. Commun., 2005, 2089–2091 RSC.
- V. Hong, N. F. Steinmetz, M. Manchester and M. G. Finn, Bioconjugate Chem., 2010, 21, 1912–1916 CrossRef CAS PubMed.
- E. V. Sokurenko, V. Vogel and W. E. Thomas, Cell Host Microbe, 2008, 4, 314–323 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and analytical data of synthesised compounds, their photochromic properties, and fluorescence images. See DOI: 10.1039/c5cc08884d |
| ‡ These authors contributed equally to the paper. |
| This journal is © The Royal Society of Chemistry 2016 |