Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thiazolium carbene catalysts for the fixation of CO2 onto amines†
Shoubhik
Das
*ab,
Felix D.
Bobbink
a,
Safak
Bulut
a,
Mylène
Soudani
a and
Paul J.
Dyson
*a
aInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland. E-mail: paul.dyson@epfl.ch
bInstitüt für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammann str. 2, Germany. E-mail: sdas@gwdg.de
First published on 18th December 2015
Abstract
The catalytic N-formylation and N-methylation of amines using CO2 as the carbon source represents a facile and sustainable approach for the synthesis of pharmaceuticals and natural products. Herein, we describe highly effective and inexpensive thiazolium carbene-based catalysts derived from vitamin B1 for the N-formylation and N-methylation of amines, using polymethylhydrosiloxane (PMHS) as a reducing agent, which operate under ambient conditions.
Continued emission of carbon dioxide has led to increased CO2 levels in the atmosphere that is impacting on climate.1 Various approaches to reduce CO2 levels in the atmosphere, such as capture of CO2 and injection deep into the earth, a practice known as carbon capture and storage (CCS),2 are under intensive investigation. To increase the viability of such processes, CO2, often regarded as waste, can be considered as an increasingly abundant chemical feedstock,3 and can be employed to generate value-added products. This approach is referred to as CCUS (carbon capture, use and storage).
The activation of CO2 is highly challenging due to its high thermodynamic stability and kinetic inertness.4 One approach is to use energy-rich substrates such as epoxides and aziridines to overcome this high activation barrier to generate heterocycles.5 Strong (energy rich) nucleophiles such as Grignard reagents, organolithium agents, organoboranes and organozinc compounds have also been used to form new C–C bonds with CO2. Often these procedures require high pressures and harsh reaction conditions, which limit practical applications of these methods in industry. Therefore, mild reaction conditions are desirable, preferably taking place at atmospheric pressure and at ambient temperatures.
Recently, N-heterocyclic carbenes (NHCs) were shown to activate CO2 under mild reaction conditions.6 Based on this discovery we investigated their applications for the synthesis of various N-methylated amines using Ph2SiH2 as a hydrogen source.7
In addition to the N-methylation of amines, N-formylation via CO2 fixation is also an attractive reaction as N-formylated compounds are key chemical intermediates for the synthesis of drugs, agrochemicals, dyes and fragrances.8 Formyl groups may also be transformed into other functional groups, as in the Vilsmeier reaction,9 allylation reactions,10etc. Their typical synthesis includes the use of chloral, formic acid, formaldehyde or formate,11 and generating them from carbon dioxide would be advantageous.
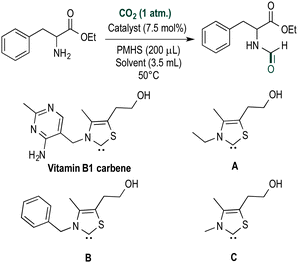
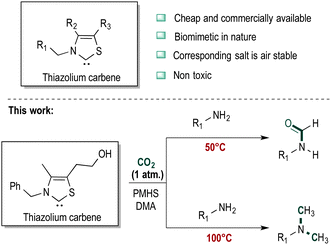
In a seminal paper ruthenium-based catalysts were used to prepare N-formylated amines using carbon dioxide and hydrogen as the reducing agent.12 Subsequent papers describe the use of palladium- and copper-based heterogeneous catalysts for this reaction.13,14 Due to the relatively harsh conditions of these reactions employing H2, other reducing agents such as hydrosilanes have broad appeal,15 especially as they are stable, easy to handle and commercially available.16 Inspired by the role of vitamin B1 in CO2 fixation in animal tissues to synthesize oxaloacetate from pyruvate, we decided to evaluate thiazolium carbenes (Scheme 1) as alternatives to NHC catalysts.17 Compared to typical NHC catalysts, thiazolium carbenes are inexpensive and non-toxic. Additionally, the corresponding salt is air stable and can be stored without the need to exclude moisture and oxygen.18 Carbene catalysts employ silanes as reductants which can also help to control chemoselectivity.19 Polymethylhydrosiloxane (PMHS) is stable, inexpensive and easily removed after reaction and, therefore, was selected for this study.
 | ||
| Scheme 1 Thiazolium carbene catalysts employed in the selective N-formylation or N-methylation of amines using CO2. | ||
Several thiazolium carbenes were investigated for the N-formylation of ethylphenylalaninate, used as a model substrate to identify and optimize the reaction and key reaction parameters (Table 1). In the presence of 7.5 mol% of B (Table 1) the corresponding ethyl formylphenylalaninate was obtained in 95% yield. The other catalysts evaluated are active, but gave lower yields of the product. The solvent used is critical, high yields were obtained in DMA and DMF in which the solubility of CO2 is high. No activity was observed in toluene, THF and acetonitrile, possibly due to the instability of the catalyst in these solvents.
| Entry | Catalyst | Silane | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: substrate (0.5 mmol), catalyst (7.5 mol%), silane (200 μL), solvent (3.5 mL), CO2 (1 atm.), 50 °C, 15 h. b Yield determined by GC using n-decane as an internal standard. c PMHS = polymethylhydrosiloxane. d DMA = N,N-dimethylacetamide. e DMF = N,N-dimethylformamide. | ||||
| 1 | Vit. B1 | PMHSc | DMAd | 58 |
| 2 | A | PMHS | DMA | 63 |
| 3 | B | PMHS | DMA | 95 |
| 4 | C | PMHS | DMA | 43 |
| 5 | B | PMHS | DMFe | 77 |
| 6 | B | PMHS | Acetonitrile | 0 |
| 7 | B | PMHS | Toluene | 0 |
| 8 | B | PMHS | THF | 0 |
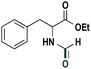
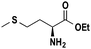
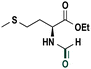
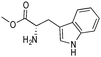
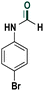
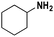
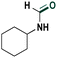
Based on the optimized reaction conditions the scope of the thiazolium carbene catalyzed N-formylation reaction was explored using catalyst B (Table 2). Aromatic, alicyclic and aliphatic amines afforded yields of up to 90%. Different amino acids such as methionine and tryptophan ethyl ester (substrates 1a–3a) reacted smoothly under the optimized reaction conditions. Moreover, para-bromo-substituted amines gave the corresponding products in 80% yield (e.g. substrate 9a) and reductive dehalogenation was not observed. Notably, the reaction may also be performed on a multigram scale without the formation of N-methylated products.
| Entry | Substrate (a) | Product (b) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: substrate (0.5 mmol), catalyst B (7.5 mol%), PMHS (200 μL), solvent (3.5 mL), CO2 (1 atm.), 50 °C, 15–24 h. b Isolated yield. c Yield determined by GC using n-decane as an internal standard for substrate 4a. | |||
| 1 |
|
|
90 |
| 2 |
|
|
87 |
| 3 |
|
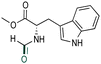
|
75 |
| 4 |
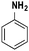
|
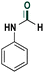
|
53c |
| 5 |
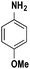
|
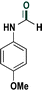
|
83 |
| 6 |
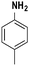
|
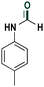
|
78 |
| 7 |
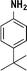
|
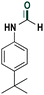
|
75 |
| 8 |
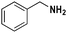
|
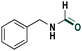
|
81 |
| 9 |
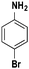
|
|
80 |
| 10 |
|
|
86 |
| 11 |
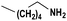
|
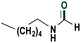
|
73 |
| 12 |

|
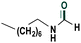
|
75 |
The formyl group is used as an amino-protecting reagent in peptide synthesis,20 subsequently being converted into isocyano acids. Formylation of peptides is achieved using formic anhydride, ammonium formate or related reagents.21 These methods have several limitations such as the thermal instability of peptides at elevated temperatures resulting in decomposition and the formation of by-products. We evaluated the N-formylation of dipeptides using catalyst B (10 mol%) and full conversion of the starting material was obtained (see the ESI†). N-Methylation of the amide bonds or other by-products was not observed. We also conducted a competition reaction in which N-methylbenzylamine and benzylamine were reacted in the same pot under the optimized conditions. The secondary amine was found to react faster (ca. 2–3 times) than the primary amine and, hence, selectivity for a substrate containing both primary and secondary amines is expected to be low.
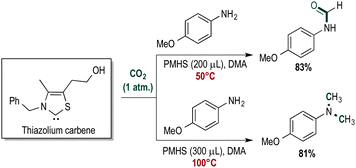
The thiazolium carbene catalyst may also be used for N-methylation reaction using CO2 combined with PMHS under slightly more forcing conditions. Numerous reports describe methylation using CO2 and hydrogen or silanes as the reducing agent.22,23 In the presence of catalyst B, 4-methoxyaniline was converted to the corresponding N,N-dimethylated aniline at 100 °C in 81% yield (Scheme 2).
N-Methylation of amines has been shown to enhance the biological activity of certain drug molecules.24 For example, inserting one or more methyl groups into a bioactive molecule can enhance lipophilicity and thereby facilitate transport through cell membranes. We successfully applied catalyst B to the N-methylation of cinacalcet (Scheme 3). The N-methylated products were purified in a facile fashion.
In summary, we have demonstrated that a bioinspired thiazolium carbene compound is an efficient catalyst for fixing CO2 onto different amines in the presence of PMHS. The lead catalyst is cheap and non-toxic. The reaction product, i.e. N-formylation versus N-methylation, may be tuned by simply changing the reaction temperature. The catalyst shows a broad substrate scope and thereby has high application in CO2 fixation reactions.
Full details are provided in the ESI†. The general procedure for the N-formylation reaction: the thiazolium salt (0.075 mmol) and sodium hydride (0.075 mmol) were dissolved in DMA (2 mL) in a 10 mL Schlenk flask and stirred for 30 min to generate carbene (7.5 mol% per mL solution). The solution was then stored in nitrogen without stirring, until the inorganic salts settled at the bottom of the flask. 1 mL of the carbene solution was transferred into a dry three neck flask (after three vacuum and CO2–purge cycles), already charged with the amine (0.5 mmol) and connected to a CO2 balloon. Next, DMA (2.5 mL) and PMHS (200–300 μL) were introduced and the reaction was monitored by TLC and GC-MS. Upon completion, the reaction mixture was filtered through celite and washed with ethyl acetate and an aqueous work up was performed, the solution dried with anhydrous sodium sulfate, and the product purified using column chromatography using ethyl acetate/pentane and 1% triethyl amine. All yields are isolated yields unless otherwise stated.
We thank The CTI Swiss Competence Center of Energy Research (SCCER) on Heat and Electricity Storage for financial support. We are also thankful to Bastien Lucas Roulier for assistance with some of the reactions.
Notes and references
- (a) T. J. Marks, et al., Chem. Rev., 2001, 101, 953 CrossRef; (b) M. Pera-Titus, Chem. Rev., 2013, 114, 1413 CrossRef PubMed.
- (a) P. S. Fennell, et al., Energy Environ. Sci., 2014, 7, 130 RSC; (b) B. Smit, J. R. Reimer, C. M. Oldenburg and I. C. Bourg, Introduction to Carbon Capture and Sequestration, Imperial College Press, London, 2014 Search PubMed; (c) H. Yang, Z. Xu, M. Fan, R. Gupta, R. B. Slimane, A. E. Bland and I. Wright, J. Environ. Sci., 2008, 20, 14 CrossRef CAS; (d) M. Mikkelsen, M. Jorgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43 RSC.
- L.-N. He, Z.-Z. Yang, A.-H. Liu and J. Gao, Advances in CO2 conversion and utilization, American Chemical Society, 2010, vol. 1056, ch. 6, p. 77 Search PubMed.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015 DOI:10.1038/ncomms6933.
- (a) W. Dai, S. Luo, S. Yin and C. Au, Appl. Catal., A, 2009, 366, 2 CrossRef CAS; (b) D. J. Darensbourg, Chem. Rev., 2007, 107, 2388 CrossRef CAS PubMed; (c) D. J. Darensbourg, R. M. Mackiewicz, A. L. Phelps and D. R. Billodeaux, Acc. Chem. Res., 2004, 37, 836 CrossRef CAS PubMed; (d) T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed; (e) M. Aresta, Carbon Dioxide as Chemical Feedstock, Wiley-VCH, Verlag GmBH, 2010 Search PubMed; (f) K. Huang, C. L. Sun and Z. J. Shi, Chem. Soc. Rev., 2011, 40, 2435 RSC.
- S. N. Riduan, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322 CrossRef CAS PubMed.
- S. Das, F. D. Bobbink, G. Laurenczy and P. J. Dyson, Angew. Chem., Int. Ed., 2014, 53, 12876 CrossRef CAS PubMed.
- (a) B. C. Chen, M. S. Bednarz, R. Zhao, J. E. Sundeen, P. Chen, Z. Shen, A. P. Skoumbourdis and J. C. Barrish, Tetrahedron Lett., 2000, 41, 5453 CrossRef CAS; (b) H. G. Grant and L. A. Summers, Aust. J. Chem., 1980, 33, 613 CrossRef CAS; (c) K. Kobayashi, S. Nagato, M. Kawakita and O. Konishi, Chem. Lett., 1995, 575 CrossRef CAS; (d) A. Tlili, E. Blondiaux, X. Frogneux and T. Cantat, Green Chem., 2015, 17, 157 RSC.
- I. M. Downie, M. J. Earle, H. Heaney and K. F. Shuhaibar, Tetrahedron, 1993, 49, 4015 CrossRef CAS.
- S. Kobayashi and K. Nishio, J. Org. Chem., 1994, 59, 6620 CrossRef CAS.
- C. J. Gerack and L. McElwee-White, Molecules, 2014, 19, 7689 CrossRef PubMed.
- L. Zhang, Z. Han, X. Zhao, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2015, 54, 6186 CrossRef CAS PubMed.
- X. Cui, Y. Zhang, Y. Deng and F. Shi, Chem. Commun., 2014, 50, 189 RSC.
- S. Kumar and L. S. Jain, RSC Adv., 2014, 4, 64277 RSC.
- (a) C. D. N. Gomes, O. Jacquet, C. Villiers, P. Thuery, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187 CrossRef PubMed; (b) Z. Yang, B. Yu, H. Zhang, Y. Zhao, G. Ji, Z. Ma, X. Gao and Z. Liu, Green Chem., 2015, 17, 4189 RSC; (c) E. Blondiaux, J. Pouessel and T. Cantat, Angew. Chem., Int. Ed., 2014, 53, 12186 CrossRef CAS PubMed; (d) T. V. Q. Nguyen, W. Yoo and S. Kobayashi, Angew. Chem., Int. Ed., 2015, 54, 9209 CrossRef CAS PubMed; (e) O. Jacquet, C. D. N. Gomes, M. Ephritikhine and T. Cantat, J. Am. Chem. Soc., 2012, 134, 2934 CrossRef CAS PubMed.
- (a) S. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507 CrossRef CAS PubMed; (b) S. Das, D. Addis, S. Zhou, K. Junge and M. Beller, J. Am. Chem. Soc., 2010, 132, 1770 CrossRef CAS PubMed; (c) S. Das, D. Addis, K. Junge and M. Beller, Chem. – Eur. J., 2011, 43, 12186 CrossRef PubMed; (d) S. Das, B. Wendt, K. Möller, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 1662 CrossRef CAS PubMed; (e) S. Das, Y. Li, C. Bornschein, S. Pisiewicz, K. Kiersch, D. Michalik, F. Gallou, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2015, 54, 12389 CrossRef CAS PubMed; (f) S. Das, Y. Li, K. Junge and M. Beller, Chem. Commun., 2012, 48, 10742 RSC.
- H. A. Krebs and L. V. Eggleston, Biochemistry, 1940, 34, 1983 Search PubMed.
- (a) I. Piel, M. D. Pawelczyk, K. Hirano, R. Fröhlich and F. Glorius, Eur. J. Org. Chem., 2011, 5475 CrossRef CAS; (b) O. Kuhl, Functionalized N-Heterocyclic Carbene Complexes, Wiley, 2010 Search PubMed.
- (a) N. C. Mamillapalli and G. Sekar, Chem. Commun., 2014, 50, 7881 RSC; (b) S. Hanada, E. Tsutsumi, Y. Motoyama and H. Nagashima, J. Am. Chem. Soc., 2009, 131, 15032 CrossRef CAS PubMed; (c) S. A. C. A. Sousa and A. C. Fernandes, Tetrahedron Lett., 2009, 50, 6872 CrossRef CAS.
- A. R. Day, N. Muthukumarswamy and R. J. Freer, Peptides, 1980, 1, 187 CrossRef CAS.
- G. Lajoie and J.-L. Kraus, Peptides, 1984, 5, 653 CrossRef CAS.
- (a) Y. Li, I. Sorribes, T. Yan, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 12156 CrossRef CAS PubMed; (b) K. Beydoun, T. vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2013, 52, 9554 CrossRef CAS PubMed; (c) X. Cui, Y. Zhang, Y. Deng and F. Shi, Chem. Commun., 2014, 50, 13521 RSC.
- (a) Y. Li, X. Fang, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 9568 CrossRef CAS PubMed; (b) O. Jacquet, X. Frogneux, C. D. N. Gomes and T. Cantat, Chem. Sci., 2013, 4, 2127 RSC; (c) L. Gonzalez-Sebastian, M. Flores-Alamo and J. J. Garcia, Organometallics, 2015, 34, 763 CrossRef CAS; (d) X. Frogneux, O. Jacquet and T. Cantat, Catal. Sci. Technol., 2014, 4, 1529 RSC.
- (a) R. Angell, N. M. Aston, P. Bamborough, J. B. Buckton, S. Cockreill, S. J. deBoeck, C. D. Edwards, D. S. Holmes, K. L. Jones, D. I. Laine, P. A. Patel, K. J. Smith, D. O. Somers and A. L. Walker, Bioorg. Med. Chem. Lett., 2008, 18, 4428 CrossRef CAS PubMed; (b) P. J. Coleman, J. D. Schreier, C. D. Cox, M. J. Breslin, D. B. Whitman, M. J. Bogusky, G. B. McGaughey, R. A. Bedner, W. Lamire, S. M. Doran, S. V. Fox, S. L. Garson, A. L. Gotter, C. M. Harrell, D. R. Reiss, T. Cabalu, D. Cui, T. Prueksaritanont, J. Stevens, P. L. Tannenbaum, R. Ball, J. Stellabott, S. D. Young, G. D. Hartman, C. J. Winrow and J. J. Renger, ChemMedChem, 2012, 7, 415 CrossRef CAS PubMed; (c) M. C. O. Reilly, S. A. Scott, K. A. Brown, T. H. Oguin, P. G. Thomas, J. S. Daniels, R. Morrison, H. A. Brown and C. A. Lindley, J. Med. Chem., 2013, 56, 2695 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc08741d |
| This journal is © The Royal Society of Chemistry 2016 |