Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structurally optimised BODIPY derivatives for imaging of mitochondrial dysfunction in cancer and heart cells†
Shubhanchi
Nigam
ab,
Benjamin P.
Burke
ab,
Laura H.
Davies
c,
Juozas
Domarkas
bd,
Jennifer F.
Wallis
c,
Paul G.
Waddell
c,
Jennifer S.
Waby
e,
David M.
Benoit
a,
Anne-Marie
Seymour
de,
Christopher
Cawthorne
be,
Lee J.
Higham
*c and
Stephen J.
Archibald
*ab
aDepartment of Chemistry, University of Hull, Cottingham Road, Hull, HU6 7RX, UK. E-mail: S.J.Archibald@Hull.ac.uk
bPositron Emission Tomography Research Centre, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
cSchool of Chemistry, Newcastle University, Bedson Building, Newcastle upon Tyne, NE1 7RU, UK
dCentre for Cardiovascular and Metabolic Research, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
eSchool of Biological, Biomedical and Environmental Sciences, University of Hull, Cottingham Road, HU6 7RX, UK
First published on 26th April 2016
Abstract
The structural features required for mitochondrial uptake of BODIPY-based optical imaging agents have been explored. The first derivatives of this class of dyes shown to have mitochondrial membrane potential-dependent uptake in both cancer and heart cells have been developed.
Mitochondrial membrane potential (MMP) plays a key role in cardiac failure and cancer.1 The electron transport chain contained within the impermeable inner mitochondrial membrane gives rise to a negative membrane potential (ca. −150 mV to −170 mV) in healthy cells.2,3 In cancerous and ischaemic heart cells, mitochondrial dysfunction can substantially disrupt the MMP4,5 resulting in up to a tenfold increase in accumulation of MMP-dependent compounds.2,3 During apoptotic or necrotic cell death, complete membrane depolarisation occurs and no uptake is observed.6,7 Lipophilic cations carrying a positive charge can pass through lipid bilayers in the mitochondria and accumulate proportionally to the change in membrane potential gradient.2 Optical agents have been developed for MMP-dependent imaging, with many derivatives based on rhodamine and tetramethylrosamine structures, with the highly effective Mitotracker® series now in common use.2,8
Boron-dipyrromethene (BODIPY) type structures are regularly used in optical biomedical imaging due to their tunable wavelength emission, photostability, remarkable brightness and biological media compatibility.9–12 Recently, the use of BODIPYs has been further extended by the incorporation of the positron emitting radioisotope, fluorine-18, resulting in multimodal positron emission tomography (PET)/optical imaging agents.13–15 Formation of BODIPY based PET/optical multimodal imaging can be achieved via B–F bond formation or modification of the structural backbone to incorporate the radiolabel.
To date, there have been no reports of BODIPY-based agents capable of imaging both cancer and heart cells in a MMP-dependent manner. Phosphonium-based lipophilic cations have been receiving interest, most notably in the development of PET imaging agents.16–21 Recently, Yuan et al. described a BODIPY triphenylphosphonium-based lipophilic cation designed for multimodal imaging which did not show MMP-dependent mitochondrial uptake.20 The development of MMP-dependent tracers would offer the capability for detection of mitochondrial dysfunction in cardiac disease and cancer together with the potential for monitoring of therapeutic responses.
Our approach in designing MMP dependent BODIPY derivatives is to form phosphonium cations directly on the lipophilic BODIPY backbone and study their chemical and biological characteristics. We have previously developed highly fluorescent, air-stable BODIPY-based phosphanes22 carrying either dicyclohexyl and diphenyl substituents.23 Methylation with methyl trifluoromethanesulfonate gave phosphonium derivatives 1 and 2 (see Fig. 1 and ESI†).
Mitochondrial uptake studies of 1 and 2 were carried out using confocal microscopy in human breast cancer cells (MCF-7) and rat cardiomyocytes (H9c2). Mitotracker deep red (MDR) was used for dye co-localisation studies as its emission wavelength (665 nm) is sufficiently different from that of 1 and 2 (532 nm) to allow independent detection. Confocal microscopy confirmed mitochondrial localisation of both 1 and 2 (see Fig. 2 and ESI† for further details). Overlap coefficients identified that both tracers showed similarly high mitochondrial localisation in H9c2 cells, however the overlap coefficient was 36% higher in MCF-7 cells for 1, indicating that it has significantly greater potential as a specific imaging agent.
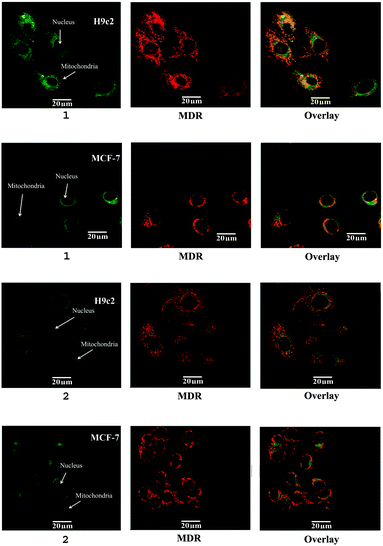 | ||
| Fig. 2 Confocal microscopy of 1 and 2 (left) in MCF-7 and H9c2 cell lines with mitotracker deep red (MDR, centre), overlaid mitochondrial co-localisation (right). | ||
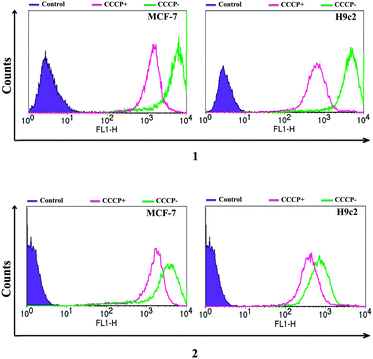
Imaging agents with MMP-dependent uptake offer an ideal tool to probe mitochondrial function. To establish the mitochondrial membrane potential specific uptake of 1 and 2, a flow cytometry study was carried out in the presence of carbonyl cyanide m-chlorophenylhydrazone (CCCP), a protonophore which eliminates the mitochondrial membrane potential (see Fig. 3 and ESI† for further details).24,25 Both tracers showed a decrease in mean fluorescent intensity (MFI) upon CCCP induced MMP depolarisation in both cell lines. 1 showed a greater than 70% decrease in MCF-7 and H9c2 cell lines, whereas 2 showed a smaller 38% and 58% decrease in MCF-7 and H9c2 cells respectively.
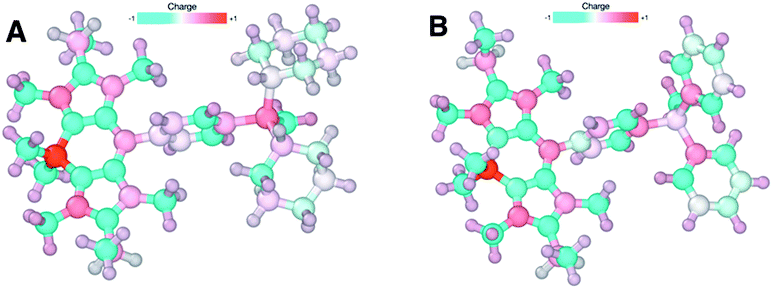
The vast majority of lipophilic cations used in molecular imaging are based on triphenylphosphonium ions.3,16,18,19 In this study, we have designed and synthesised a triphenylphosphonium BODIPY structure (2) along with a cyclohexyl derivative (1) to explore the structural features necessary for MMP-dependent uptake. Both the mitochondrial localisation determined by confocal microscopy and the flow cytometry MMP-dependent uptake studies demonstrate that the replacement of two phenyl groups for two cyclohexyl groups cause an increase in MMP dependent, mitochondrial specific uptake. DFT calculations were carried out on both structures to determine the degree of delocalisation of electron density. Our calculations show (see Fig. 4 and ESI† for further details) that the charge is localised on the phosphorus atom for the cyclohexyl derivative (1) while it is mainly delocalised on the aromatic ligands for the triphenylphosphonium structure (2). The effect of the degree of lipophilicity on the uptake of lipophilic cations has been partially explored in previous studies, generally when describing selectivity between cancer and cardiac uptake.16 The lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) values of 1 and 2 were determined to be 3.19 and 2.80 respectively, revealing a significant increase in lipophilicity for the cyclohexyl derivative.
P) values of 1 and 2 were determined to be 3.19 and 2.80 respectively, revealing a significant increase in lipophilicity for the cyclohexyl derivative.
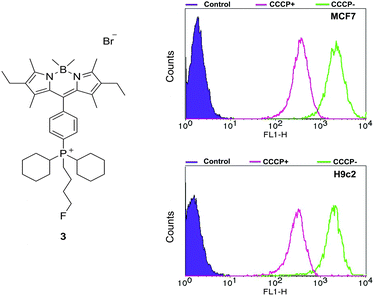
To validate the strategy and open opportunities for future PET/optical imaging agents, we have replaced methyl trifluoromethanesulfonate in the final step methylation reaction of the dicyclohexyl derivative with 1-bromo-4-fluorobutane. The structure of the formed product, 3, was confirmed by NMR and X-ray crystallography (see ESI†) and is the cold standard of a potential future radiolabelled product. The fluorine-18 analogue can be prepared via conventional nucleophilic substitution in a one step procedure or directly from 1 in a 2-step radiolabelling protocol.26,27
Flow cytometry studies on 3, carried out using an identical protocol to that used to analyse 1 and 2, demonstrated that this fluoride substituted derivative behaves in an analogous fashion. Replacement of the methyl group in 1 with a fluorobutyl leads to an increase in MMP dependent uptake, with CCCP causing decreases in uptake of 84% and 83% in MCF-7 and H9c2 cells respectively (see Fig. 5), thus validating this approach for the design of multimodal optical/PET imaging agents.
In conclusion, we have developed novel BODIPY based agents for mitochondrial imaging. These agents, for the first time, have been shown to be taken up in mitochondria in a mitochondrial membrane potential-dependent manner in both cancer and heart cells. The new structural class, with the phosphonium grafted directly on the BODIPY backbone, offers the opportunity to reduce non-specific binding by optimising the lipophilicity of a dicyclohexyl derviative with shielded localised charge. This work validates the structural features required to develop MMP-dependent PET/optical multimodal imaging via fluorine-18 radiolabelling. Studies are currently underway to develop these agents for in vivo use.
We gratefully acknowledge the Daisy Appeal Charity for funding (Grant: DAhul0211), the University of Hull for a fee reduction scholarship for SN, Newcastle University for a studentship LHD, EPSRC and High Force Research Ltd for financial support of JFW and EPSRC for a LJH Fellowship (EP/G005206/1). We thank Dr Assem Allam for his generous donation and ongoing support to the PET Research Centre at the University of Hull. The authors wish to thank the EPSRC National Mass Spectrometry Service at Swansea.
Notes and references
- S. Fulda, L. Galluzzi and G. Kroemer, Nat. Rev. Drug Discovery, 2010, 9, 447–464 CrossRef CAS PubMed.
- M. P. Murphy, Biochim. Biophys. Acta, 2008, 1777, 1028–1031 CrossRef CAS PubMed.
- J. S. Modica-Napolitano and J. R. Aprille, Adv. Drug Delivery Rev., 2001, 49, 63–70 CrossRef CAS PubMed.
- J. S. Modica-Napolitano and J. R. Aprille, Cancer Res., 1987, 47, 4361–4365 CAS.
- B. Kadenbach, R. Ramzan, R. Moosdorf and S. Vogt, Mitochondrion, 2011, 11, 700–706 CrossRef CAS PubMed.
- H. W. Strauss and H. Schoeder, J. Am. Coll. Cardiol. Img., 2012, 5, 293–296 CrossRef PubMed.
- V. Ganitkevich, S. Reil, B. Schwethelm, T. Schroeter and K. Benndorf, Circ. Res., 2006, 99, 165–171 CrossRef CAS PubMed.
- M. Poot, Y. Z. Zhang, J. A. Kramer, K. S. Wells, L. Jones, D. K. Hanzel, A. G. Lugade, V. L. Singer and R. P. Haughland, J. Histochem. Cytochem., 1996, 44, 1363–1372 CrossRef CAS PubMed.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- J. Karolin, L. B. A. Johansson, L. Strandberg and T. Ny, J. Am. Chem. Soc., 1994, 116, 7801–7806 CrossRef CAS.
- L. H. Davies, B. B. Kasten, P. D. Benny, R. L. Arrowsmith, H. Ge, S. I. Pascu, S. W. Botchway, W. Clegg, R. W. Harrington and L. J. Higham, Chem. Commun., 2014, 50, 15503–15505 RSC.
- Z. Li, T. P. Lin, S. Liu, C. W. Huang, T. W. Hudnall, F. P. Gabbai and P. S. Conti, Chem. Commun., 2011, 47, 9324–9326 RSC.
- J. A. Hendricks, E. J. Keliher, D. Wan, S. A. Hilderbrand, R. Weissleder and R. Mazitschek, Angew. Chem., Int. Ed., 2012, 51, 4603–4606 CrossRef CAS PubMed.
- B. P. Burke, G. S. Clemente and S. J. Archibald, Contrast Media Mol. Imaging, 2015, 10, 96–110 CrossRef CAS PubMed.
- Y. Zhou and S. Liu, Bioconjugate Chem., 2011, 22, 1459–1472 CrossRef CAS PubMed.
- X. Yan, Y. Zhou and S. Liu, Theranostics, 2012, 2, 988–998 CrossRef CAS PubMed.
- D.-Y. Kim, H.-S. Kim, L. Uyenchi Nguyen, S. N. Jiang, H.-J. Kim, K.-C. Lee, S.-K. Woo, J. Chung, H.-S. Kim, H.-S. Bom, K.-H. Yu and J.-J. Min, J. Nucl. Med., 2012, 53, 1779–1785 CrossRef CAS PubMed.
- Z. Zhao, Q. Yu, T. Mou, C. Liu, W. Yang, W. Fang, C. Peng, J. Lu, Y. Liu and X. Zhang, Mol. Pharmaceutics, 2014, 11, 3823–3831 CrossRef CAS PubMed.
- H. Yuan, H. Cho, H. H. Chen, M. Panagia, D. E. Sosnovik and L. Josephson, Chem. Commun., 2013, 49, 10361–10363 RSC.
- B. P. Burke, P. Greenman, A. M. Smith and S. J. Archibald, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o3202 CAS.
- L. H. Davies, B. Stewart, R. W. Harrington, W. Clegg and L. J. Higham, Angew. Chem., Int. Ed., 2012, 51, 4921–4924 CrossRef CAS PubMed.
- L. H. Davies, R. W. Harrington, W. Clegg and L. J. Higham, Dalton Trans., 2014, 43, 13485–13499 RSC.
- H. Steen, J. G. Maring and D. K. F. Meijer, Biochem. Pharmacol., 1993, 45, 809–818 CrossRef CAS PubMed.
- M. L. R. Lim, T. Minamikawa and P. Nagley, FEBS Lett., 2001, 503, 69–74 CrossRef CAS PubMed.
- P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998–9033 CrossRef CAS PubMed.
- F. Buckingham and V. Gouverneur, Chem. Sci., 2016, 7, 1645–1652 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, fluorescence spectra, partition coefficient determination, confocal microscopy details, flow cytometry details and computational chemistry details. CCDC 1474862. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc08325g |
| This journal is © The Royal Society of Chemistry 2016 |