The reduction of graphene oxide with hydrazine: elucidating its reductive capability based on a reaction-model approach†
Chun Kiang
Chua
and
Martin
Pumera
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: pumera.research@gmail.com
First published on 3rd November 2015
Abstract
We have performed an experimental investigation on the effects of hydrazine treatment on graphene oxide via a reaction-model approach. Hydrazine was reacted with small conjugated aromatic compounds containing various oxygen functional groups to mimic the structure of graphene oxide. The hydroxyl and carboxylic groups were not readily removed while carbonyl groups reacted with hydrazine to form the corresponding hydrazone complexes. In the presence of adjacent hydroxyl groups, carboxyl groups underwent thermal decarboxylation.
It has been a decade since graphene was successfully isolated.1 Graphene promises the development of state-of-the-art devices, ranging from electronics,2 sensors,3 and healthcare devices4 to energy storage and conversion devices,5 due to its exceptional structural, physical and electronic properties.6 In order to achieve this, numerous synthesis strategies have been highlighted over the past years to produce bulk quantity of high quality graphene. These strategies include top-down and bottom-up methods. The bottom-up method produces high quality graphene sheets but suffers from high cost and low yield. On the other hand, the top-down method realises the possibility of bulk production of graphene but the integrity of the sp2 carbon network is often compromised.
In a typical top-down approach based on chemical reaction, graphite is first oxidised with strong acids and oxidants to produce graphite oxide, an intermediate which consists of oxygen-containing groups, including hydroxyl, epoxide, carbonyl and carboxyl groups.7 Several models of graphite oxide have been proposed, with the Lerf–Klinowski model (Fig. 1) being the most frequently represented in the literature.8 A recent study also highlighted graphite oxide as a bi-component material consisting of relatively unoxidised sheets and highly oxidized small aromatics,9 where the latter can be removed upon base wash, chemical reduction or ultrasonication treatment.10 Nevertheless, graphite oxide is typically ultrasonicated and reduced to yield graphene sheets.8b One of the most common reducing agents applied for the reduction of graphene oxide is hydrazine.11 In contrast to how hydrazine is applied as a reducing agent in the context of graphene oxide, hydrazine is typically applied in organic chemistry only as a reducing agent for the Wolff–Kishner reaction (conversion of ketone to alkane) and as a nucleophile for most other types of reactions.12 It is, as such, difficult to establish a direct link between the functions of hydrazine in organic chemistry and in the chemistry of graphene oxide. This has thus encouraged the development of experimental and computational investigations on the working mechanisms of hydrazine on graphene oxide. Ruoff and co-workers have proven experimentally with the usage of solid state NMR and XPS analyses of 13C- and 15N-labelled hydrazine-reduced graphene that five-membered pyrazole or pyrazoline rings are formed at the edges of the graphene sheets.13 On the other hand, density functional theory was applied to investigate the proposed mechanisms for the reduction of the epoxide group with hydrazine.14 Nagase and co-workers further investigated the mechanisms and effects of hydrazine (25 °C) and thermal annealing (700–1200 °C) treatments on various small fragments of graphene sheets containing hydroxyl, epoxide, carbonyl and carboxylic groups.14b The computational study showed that hydrazine treatment at room temperature does not spontaneously remove the hydroxyl, epoxide, carbonyl and carboxylic groups present at the edge plane of graphene sheets. Instead, the epoxide and hydroxyl groups present in the basal plane are removed favourably.
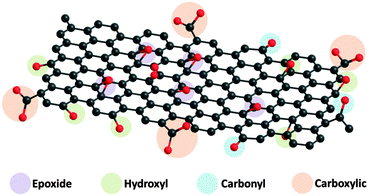 | ||
| Fig. 1 Oxygen functional groups found on graphene oxide, including epoxide, hydroxyl, carbonyl and carboxyl groups. | ||
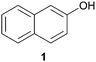
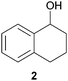
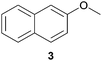
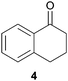
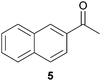
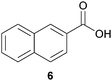
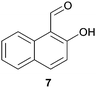
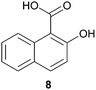
In this work, the effects of hydrazine as a reducing agent, in the context of graphene oxide reduction, are investigated experimentally on small conjugated aromatic compounds. While graphene oxide should be considered as a highly conjugated macromolecule and its chemistry, as such, should be treated differently, it is nevertheless worthwhile to examine the possible chemical transformations occurring in simple organic molecules upon treatment with hydrazine. In view of this, small conjugated aromatic compounds containing specific oxygen functional groups (i.e., hydroxyl, carbonyl, carboxylic, methoxy) were treated with hydrazine and analysed for possible functional group transformations. These include 2-naphthol (1), 1,2,3,4-tetrahydro-1-naphthol (2), 2-methoxynaphthalene (3), 1-tetralone (4), 2-acetonapthone (5), 2-naphthoic acid (6), 2-hydroxy-1-naphthaldehyde (7) and 2-hydroxy-1-naphthoic acid (8) (see Table 1). Control experiments which excluded the addition of hydrazine were also performed simultaneously in order to determine the effects of thermal treatment on compounds 1–8. This work provides a basis for future investigations on more complex conjugated molecules as models to understand the reductive effects of hydrazine on graphene oxide.
In a typical reaction of graphene oxide with hydrazine, hydrazine was added into a dispersion of graphene oxide in water and subsequently heated to 100 °C for 24 hours. However, in order to ensure the complete dissolution of the conjugated aromatic compounds applied as model molecules in this study, the procedure was modified by changing the dispersion medium from water to a mixture of DMF and water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This new procedure was applied for the reduction of graphene oxide, whereby graphene oxide was observed to agglomerate into clusters over the course of the reaction, which is consistent with typical observations.
1). This new procedure was applied for the reduction of graphene oxide, whereby graphene oxide was observed to agglomerate into clusters over the course of the reaction, which is consistent with typical observations.
Further X-ray photoelectron spectroscopy (XPS) analyses indicated a well-reduced graphene sample (Fig. 2). The survey scan showed the peaks of C1s, N1s and O1s in the ratio of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The derived C/O ratio of 6.9 was typical for hydrazine-reduced graphene oxide.15 Moreover, the C1s high-resolution core-level spectrum showed a sharp peak at 284.5 eV (C
5. The derived C/O ratio of 6.9 was typical for hydrazine-reduced graphene oxide.15 Moreover, the C1s high-resolution core-level spectrum showed a sharp peak at 284.5 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond) with extended tailing into the region of higher binding energy. The presence of C–C, C–O, C
C bond) with extended tailing into the region of higher binding energy. The presence of C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–C
O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and π–π* peaks was obvious as well. Since the revised protocol provided a good extent of reduction on the graphene oxide, compounds 1–8, which were mainly naphthalene-based, were subjected to hydrazine treatment at 100 °C for 24 hours in a DMF/water medium. Alongside that, control experiments performed in the absence of hydrazine were conducted to determine the effects of thermal treatment on the compounds. A summary of hydrazine and the thermal effects on compounds 1–8 is listed in Table 1.
O and π–π* peaks was obvious as well. Since the revised protocol provided a good extent of reduction on the graphene oxide, compounds 1–8, which were mainly naphthalene-based, were subjected to hydrazine treatment at 100 °C for 24 hours in a DMF/water medium. Alongside that, control experiments performed in the absence of hydrazine were conducted to determine the effects of thermal treatment on the compounds. A summary of hydrazine and the thermal effects on compounds 1–8 is listed in Table 1.
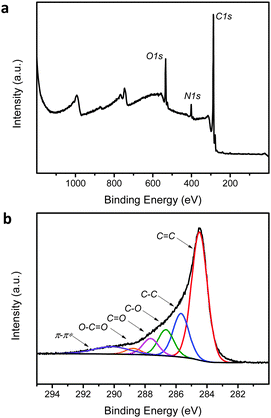 | ||
| Fig. 2 X-ray photoelectron spectra of hydrazine-reduced graphene oxide based on the (a) survey and (b) high-resolution C1s core-level analyses. | ||
The possible events of hydrazine- and thermal-induced dehydroxylation were investigated with compounds 1 and 2. Based on previous theoretical studies by Nagase and co-workers, the hydroxyl groups attached to the inner aromatic domains of graphene oxide were expected to dissociate or migrate to the edges of the aromatic domains, which thereafter would dissociate off upon thermal treatment (700–1200 °C).14b In this study, both compounds 1 and 2 represented hydroxyl groups at the edges of graphene oxide sheets, with compound 1 being positioned in an aromatic domain while compound 2 being situated in a non-aromatic domain. When compound 1 was reacted with hydrazine, no obvious dehydroxylation was observed as only the starting material was detected by NMR analysis. However, the presence of naphthalene was occasionally observed at a very low proportion by NMR analysis over the span of several repetitions. Similarly, treatment of compound 2 with hydrazine did not result in any dehydroxylation effect although slight traces of undetermined complexes were observed by NMR analysis in very small proportions. In an effort to study the possible demethoxylation or demethylation processes occurring in the aromatic domains, compound 3 was treated with hydrazine but it remained unreactive.
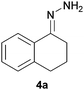
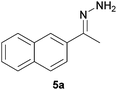
Subsequently, the decarbonylation process was examined with compounds 4 and 5. Similar to dehydroxylation, Nagase and co-workers have theoretically proven that carbonyl groups were resistant to decarbonylation under both hydrazine (25 °C) and thermal treatment (700–1200 °C).14b In our case, when compound 4 was reacted with hydrazine at 100 °C for 24 hours, the starting material was fully consumed to provide the corresponding hydrazone 4a in qualitative yield. Interestingly, traces of the corresponding azine complex were also detected, which supported the possible formation of pyrazole from diketone moieties.13 Moreover, hydrazone 4a was observed to react with trace acetone to form an azine complex. As a matter of fact, while Nagase and co-workers predicted the presence of hydrazino alcohol from the reaction between the edge-terminated epoxide group and hydrazine,14b it may be useful to note that the hydrazino alcohol could further react with typical organic solvents (e.g. acetone) applied for washing hydrazine-reduced graphene materials. Further studies on the reaction of 2-tetralone and hydrazine provided a mixture consisting of mainly decomposed materials. As for compound 5 which consisted of a free carbonyl group, the corresponding hydrazone 5a was obtained in 85% yield. While decarboxylation was predicted by Nagase and co-workers to occur slowly at room temperature14b and even at 100–150 °C,11,16 decarboxylation was not observed in this study when compound 6 was treated with hydrazine at 100 °C for 24 hours.
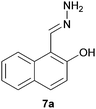
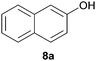
We extrapolated the study to conjugated aromatic compounds containing a mixture of oxygen functional groups, specifically compounds 7 and 8, which consisted of carbonyl/hydroxyl and carboxylic/hydroxyl functional groups, respectively. In the case of compound 7, the corresponding hydrazone 7a was obtained as a major product (84%) when treated with hydrazine. Thermal treatment did not result in any functional group transformation. As for compound 8, thermal treatment caused a decarboxylation process toward the formation of 2-naphthol (8a, 80%), while no further reactions were observed in the presence of hydrazine. Thermal decarboxylation was however not observed in compound 6, which suggested a possible hydroxyl group effect on this process.
In summary, the reactions of hydrazine with various conjugated aromatic compounds were investigated based on a reaction-model approach to understand the reactivity of hydrazine on graphene oxide. This study showed that hydroxyl and carboxylic groups were not readily removed while carbonyl groups formed the corresponding hydrazone complexes. These observations may be anticipated from the organic chemistry point of view, but it is nevertheless important to extend the investigations to more complex conjugated aromatic compounds to further understand the underlying effects of hydrazine treatment on graphene oxide. Furthermore, the phenomenon of the thermal decarboxylation of a carboxyl group containing an adjacent hydroxyl group could provide useful insights into the observed precipitation of graphene during the reduction of graphene oxide with hydrazine. This work highlights the importance of using small organic molecules as models to study the reactions occurring on graphene oxide.
This work was supported by a Tier 2 grant (MOE2013-T2-1-056; ARC 35/13) from the Ministry of Education, Singapore.
Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- N. O. Weiss, H. L. Zhou, L. Liao, Y. Liu, S. Jiang, Y. Huang and X. F. Duan, Adv. Mater., 2012, 24, 5782–5825 CrossRef CAS PubMed.
- A. Ambrosi, C. K. Chua, A. Bonanni and M. Pumera, Chem. Rev., 2014, 114, 7150–7188 CrossRef CAS PubMed.
- K. V. Krishna, C. Menard-Moyon, S. Verma and A. Bianco, Nanomedicine, 2013, 8, 1669–1688 CrossRef CAS PubMed.
- (a) M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 RSC; (b) F. Bonaccorso, L. Colombo, G. H. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- (a) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed; (b) K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- (a) L. Staudenmaier, Ber. Dtsch. Chem. Ges., 1898, 31, 1481–1487 CrossRef CAS; (b) U. Hofmann and E. Konig, Z. Anorg. Allg. Chem., 1937, 234, 311–336 CrossRef CAS; (c) W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; (d) D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- (a) A. Lerf, H. Y. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS; (b) C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- J. P. Rourke, P. A. Pandey, J. J. Moore, M. Bates, I. A. Kinloch, R. J. Young and N. R. Wilson, Angew. Chem., 2011, 123, 3231–3235 CrossRef.
- (a) H. R. Thomas, S. P. Day, W. E. Woodruff, C. Vallés, R. J. Young, I. A. Kinloch, G. W. Morley, J. V. Hanna, N. R. Wilson and J. P. Rourke, Chem. Mater., 2013, 25, 3580–3588 CrossRef CAS; (b) A. Bonanni, A. Ambrosi, C. K. Chua and M. Pumera, ACS Nano, 2014, 8, 4197–4204 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, John Wiley & Sons, New Jersey, 6th edn, 2007 Search PubMed.
- S. Park, Y. Hu, J. O. Hwang, E.-S. Lee, L. B. Casabianca, W. Cai, J. R. Potts, H.-W. Ha, S. Chen, J. Oh, S. O. Kim, Y.-H. Kim, Y. Ishii and R. S. Ruoff, Nat. Commun., 2012, 3, 638 CrossRef PubMed.
- (a) M. C. Kim, G. S. Hwang and R. S. Ruoff, J. Chem. Phys., 2009, 131, 064704 CrossRef PubMed; (b) X. F. Gao, J. Jang and S. Nagase, J. Phys. Chem. C, 2010, 114, 832–842 CrossRef CAS.
- C. K. Chua and M. Pumera, J. Mater. Chem., 2012, 22, 23227–23231 RSC.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and NMR spectra. See DOI: 10.1039/c5cc08170j |
| This journal is © The Royal Society of Chemistry 2016 |