Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design and synthesis of a new chromophore, 2-(4-nitrophenyl)benzofuran, for two-photon uncaging using near-IR light†
Naomitsu
Komori
a,
Satish
Jakkampudi
ab,
Ryusei
Motoishi
a,
Manabu
Abe
*abc,
Kenji
Kamada
*d,
Ko
Furukawa
e,
Claudine
Katan
 *f,
Wakako
Sawada
g,
Noriko
Takahashi
g,
Haruo
Kasai
bg,
Bing
Xue
bh and
Takayoshi
Kobayashi
bh
*f,
Wakako
Sawada
g,
Noriko
Takahashi
g,
Haruo
Kasai
bg,
Bing
Xue
bh and
Takayoshi
Kobayashi
bh
aDepartment of Chemistry, Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-hiroshima, Hiroshima 739-8526, Japan. E-mail: mabe@hiroshima-u.ac.jp
bJST-CREST, K’s Gobancho, 7, Gobancho, Chiyoda-ku, Tokyo 102-0076, Japan
cResearch Centre for Smart Materials, Hiroshima University, 1-3-1 Kagamiyama, Higashi-hiroshima, Hiroshima 739-8526, Japan
dIFMRI, National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan. E-mail: k.kamada@aist.go.jp
eCentre for Instrumental Analysis, Niigata University, 8050 Ikarashi 2-no-cho, Nishi-ku, Niigata 950-2181, Japan
fInstitut des Sciences Chimiques de Rennes, UMR 6226 CNRS-Universite Rennes 1, 35042 Rennes, France. E-mail: claudine.katan@univ-rennes1.fr
gLaboratory of Structural Physiology, CDBIM, Graduate School of Medicine, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan
hAdvanced Ultrafast Laser Research Center, The University of Electro-Communications, Chofugaoka 1-5-1, Chofu, Tokyo 182-8585, Japan
First published on 21st October 2015
Abstract
A new chromophore, 2-(4-nitrophenyl)benzofuran (NPBF), was designed for two-photon (TP) uncaging using near-IR light. The TP absorption (TPA) cross-sections of the newly designed NPBF chromophore were determined to be 18 GM at 720 nm and 54 GM at 740 nm in DMSO. The TP uncaging reaction of a caged benzoate with the NPBF chromophore quantitatively produced benzoic acid with an efficiency (δu) of ∼5.0 GM at 740 nm. The TP fragmentation of an EGTA unit was observed with δu = 16 GM. This behavior makes the new chromophore a promising TP photoremovable protecting group for physiological studies.
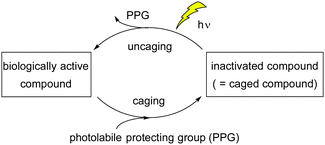
Caged compounds1 have attracted considerable interest in physiological studies, e.g. in neuroscience, due to their many applications.2–5 These compounds, in which biologically active molecules are inactivated by photoremovable protecting groups (PPGs),6 can uncage (=release) bioactive molecules upon photolysis (Fig. 1).4 Spatial and temporal control of the uncaging process allows detailed investigation of the role of bioactive molecules in vivo.
Two-photon absorption (TPA) by near-IR irradiation of light (approximately 700–1300 nm) has been increasingly used for photolysis in biological studies because most living tissues have low absorption and scattering in this wavelength region.5,7 In addition, TPA enables spatially selective excitation of a small volume near the focal point of the laser beams. Thus two-photon (TP) excitation affords uncaging with higher spatial resolution, less photodamage to cells and tissues, and deeper penetration depth than one-photon (OP) excitation processes.5,7 However, to work under the physiological conditions, a high uncaging efficiency is required. This efficiency is defined as the uncaging quantum yield (ϕu) multiplied by the excitation probability.8 The excitation probability can be expressed using the molar extinction coefficient (ε) for OP excitation or using the TPA cross-section (σ2) for TP excitation. From this convention, TP-efficiency (δu = ϕuσ2) has the same units as the TPA cross section (GM). For biological applications, the minimum δu threshold value currently accepted is 3 GM.9,10 This calls for development of biocompatible caged compounds with a sizeable δu to ensure efficient release with tissue-permeable near-IR irradiation, providing valuable targets for applications in in vivo physiological studies.
Several PPGs with TPA character (TP-PPGs) have been reported so far.11,12 For instance, in 2006, Ellis-Davis and co-workers reported a nitrodibenzofuran (NDBF) skeleton with a planar biphenyl structure (Fig. 2). A caged calcium with the NDBF chromophore has a high ε (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1) and ϕu (0.7) in the OP excitation process,12 but a low δu (∼0.6 GM) in an aqueous buffer solution at 720 nm photolysis. Such a small δu is not surprising, given that NDBF has a dipolar character with an OPA maximum at 331 nm,13 that predicts the lowest energy TPA maximum near 660 nm, far away from 720 nm.14a This prompted us to design a new chromophore having a red-shifted absorption band.
400 M−1 cm−1) and ϕu (0.7) in the OP excitation process,12 but a low δu (∼0.6 GM) in an aqueous buffer solution at 720 nm photolysis. Such a small δu is not surprising, given that NDBF has a dipolar character with an OPA maximum at 331 nm,13 that predicts the lowest energy TPA maximum near 660 nm, far away from 720 nm.14a This prompted us to design a new chromophore having a red-shifted absorption band.
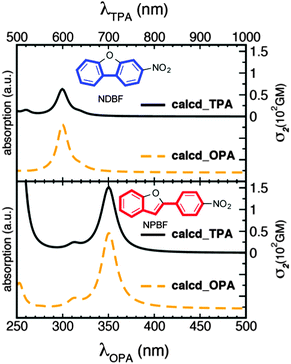 | ||
| Fig. 2 Calculated OPA (dashed lines) and TPA (solid lines) spectra of NDBF (top panel) and NPBF chromophores (bottom panel). | ||
The extension of the π-conjugation system is an efficient way to bathochromically shift the absorption maximum and also improve the TP properties of chromophores.15 At the same time, one may want to retain a simple synthetic scheme.
In our previous study,16 a stilbene skeleton was chosen for its inherently high TPA cross-section of 12 GM at 514 nm, in spite of its small π-conjugated portion.17,18 To avoid cis–trans isomerization in the excited state, the 1,2-dihydronaphthalene cyclic structure was chosen for the TP-uncaging reaction.16
We report herein the design and synthesis of a new TP-PPG with a 2-(4-nitrophenyl)benzofuran (NPBF) chromophore. The dipolar character of the donor–π–acceptor skeleton with a cyclic stilbene structure is promising to increase the TPA cross-section (Fig. 2).14,15 For comparison, the TP uncaging reaction of a caged benzoate 5 with NPBF is also investigated under irradiation in the 700–760 nm range.
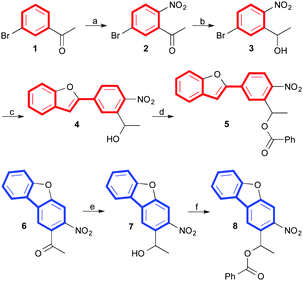
First, the OPA and TPA spectra of the parent NPBF were computed at the TD-B3LYP/6-31G*//HF/6-31G* level of theory and compared with those of the NDBF core (Fig. 2). This level of theory has been shown to provide good predictions for structure–TPA relationships.14a A detailed discussion of the accuracy of the calculated TPA cross sections can be found in the ESI.† As expected, the extended π-conjugation system of the NPBF chromophore led to a sizable TPA cross-section of about 150 GM at a longer wavelength of approximately 700 nm in gas phase.19 On the other hand, the NDBF chromophore showed a relatively small TPA cross-section of about 50 GM at a shorter wavelength of about 600 nm. The NPBF calculations prompted us to synthesize the caged benzoate 5 and investigate its TP photochemical reactivity. To compare the TP uncaging reaction of the NPBF chromophore with the NDBF chromophore, caged benzoate 8 was also prepared.
The syntheses of caged benzoates 5 and 8 are summarized in Scheme 1. The key intermediate 1-(5-bromo-2-nitrophenyl)ethane-1-ol 3 was prepared using a modified method from our previous work, starting with 3-bromoaceto-phenone 1.16 Next, 1-(5-(benzofuran-2-yl)-2-nitrophenyl)ethane-1-ol 4, a compound exhibiting a rigid stilbene structure, was synthesized in 95% yield by reacting 3 with commercially available 2-benzofuranboronic acid through a Suzuki–Miyaura cross-coupling.20 1-(3-Nitrodibenzo[b,d]furan-2-yl)ethane-1-one 6 was prepared according to a previously reported synthetic method,12 and was then rapidly reduced with NaBH4 to provide the corresponding alcohol 7, exhibiting a NDBF core. Finally, the benzoic acid (BA) moiety was introduced to alcohols 4 and 7 to provide the caged benzoates NPBF-BA 5 and NDBF-BA 8 in 64% and 56% yields, respectively.21
The absorption spectra of 5 and 8 were measured in DMSO (Fig. S14, ESI†). The absorbance maxima of 5 and 8 were found at 364 nm (ε = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and 320 nm (ε = 9000 M−1 cm−1), respectively. The red shift and increased absorbance of 5 were found to be consistent with the theoretical predictions.
000 M−1 cm−1) and 320 nm (ε = 9000 M−1 cm−1), respectively. The red shift and increased absorbance of 5 were found to be consistent with the theoretical predictions.
The photolytic release of carboxylic acid22 from compounds 5 and 8 was investigated by irradiation with a Xe-lamp at 360 ± 10 nm in DMSO-d6 (Fig. 3 and 4). The reaction was monitored using 1H NMR (400 MHz). The NMR signals of benzoic acid in the aromatic region are shown in Fig. 3e and 4e, with the overall time evolution of the reaction demonstrating a quantitative uncaging, larger than 90%. The uncaging quantum yields ϕu were determined in DMSO using the photochemical actinometer ferrioxalate coupled with high-performance liquid chromatography (HPLC), which led to values of 0.09 and 0.21 for 5 and 8, respectively. Although the reported uncaging quantum yield of NDBF-EGTA12 is 0.7, that of the measured NDBF-BA species is 0.21, despite having the same core. This indicates substituent effects on the benzyl position of the o-nitrobenzyl caging groups.4 The OP photochemical efficiency (ε360 × ϕu) of the uncaging reaction of 5 (ε360 = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) at 360 nm was 1080, which was nearly the same as the value of 1071 obtained for 8 (ε360 = 5100).
000) at 360 nm was 1080, which was nearly the same as the value of 1071 obtained for 8 (ε360 = 5100).
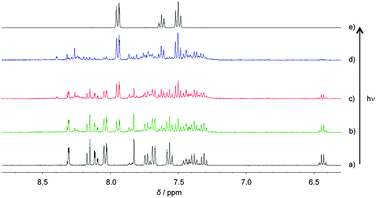 | ||
| Fig. 3 1H NMR spectra of compound 5 in DMSO-d6 (a) before and after (b) 6, (c) 12, (d) and 24 h of irradiation at 360 nm; (e) the 1H NMR spectrum of benzoic acid in DMSO-d6. | ||
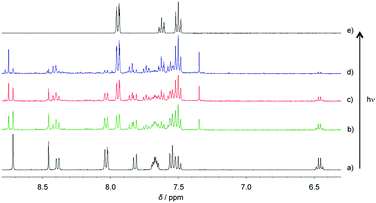 | ||
| Fig. 4 1H NMR spectra of compound 8 in DMSO-d6 (a) before and after (b) 1, (c) 4, (d) and 6 h of irradiation at 360 nm; (e) the 1H NMR spectrum of benzoic acid in DMSO-d6. | ||
The TPA cross-sections of the parent cores of NPBF and NDBF were measured at 720 nm by the Z-scan method, in which the sample was moved along the path of the laser beam and the light intensity was measured by a detector as a function of its position on the Z-axis.23 Values of 18 ± 3 and 6 ± 1 GM were obtained for the NPBF and NDBF cores, respectively, at 720 nm in DMSO. Detailed information for the determination of the values can be found in the ESI.†
TP photolysis of 5 and 8 was carried out in DMSO using 700, 710, 720, 730, 740, 750, and 760 nm light from a Ti:sapphire laser (pulse width 100 fs, 80 MHz) emitting at an average of 700 mW. The consumption of 5 and 8 and the production of benzoic acid upon photolysis were monitored by HPLC (Fig. 5).
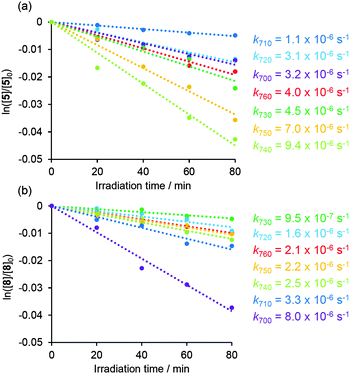 | ||
| Fig. 5 Time profile of TP uncaging of (a) 5 and (b) 8, ln([sub]/[sub]0) versus irradiation time at wavelengths of 700, 710, 720, 730, 740, 750, and 760 nm and at a power of 700 mW. | ||
As shown in Fig. 5, the TP-uncaging rates were found to vary depending on the excitation wavelength. The fastest rate of 5 (k740 = 9.4 × 10−6 s−1) was found at 740 nm while that of 8 was at 700 nm (k700 = 8.0 × 10−6 s−1). The data show the improved performance of the NPBF structure in 5 for the new chromophore. The other rate constants of TP uncaging reaction of 5 and 8 were measured in similar ways using near-IR photolyses (Fig. 5).
The rate constant of 5 at 720 nm was 3.1 × 10−6 s−1 (Fig. 5a), about one third of the value that was observed at 740 nm. Since the absolute value of the TPA cross-section of the NPBF core was 18 GM at 720 nm, the corresponding value at 740 nm can be extrapolated to be about 54 GM. This would give a δu value of 5.0 GM (54 GM × 0.09), which is higher than the threshold value of δu > 3 GM.9,10
The TPA spectra of 5 and 8 over a wavelength range of 700–760 nm were extrapolated based on the TPA cross-section values at 720 nm of the two species (Fig. 6). The new NPBF species clearly shows highly improved TPA in the near-IR region when compared to the previously reported NDBF derivatives.
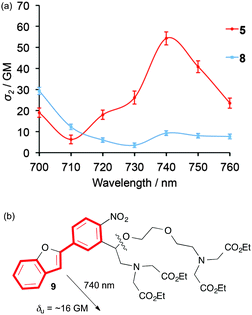 | ||
| Fig. 6 (a) TPA spectra of 5 (red line) and 8 (blue line), as determined by the relative rate constants of the corresponding uncaging reactions; (b) TP reaction of 9 at 740 nm, δu = ∼16 GM, ϕu = 0.3. | ||
The new TP-PPG-NPBF chromophore was successfully applied for the photochemical decomposition of ethylene glycol tetraacetic acid (EGTA) derivative NPBF-EGTA 9 (Fig. 6b). The EGTA unit is important for caging Ca2+.12,24 The TP-induced fragmentation of the EGTA unit was observed with δu = 16 GM (ϕu = 0.3) at 740 nm in benzene (Fig. S17 and S18, ESI†).
In conclusion, a new NPBF chromophore with TPA capability was designed and synthesized. A simple increase in the π-conjugation length and donating ability led to a bathochromic shift and a significant increase in TP sensitivity. The OP photolysis of 5 showed the quantitative release of benzoic acid with a high uncaging efficiency εϕu = 1080 at 360 ± 10 nm. Meanwhile, TP uncaging of 5 using near-IR light quantitatively produced benzoic acid with an efficiency of 5.0 GM at 740 nm, corresponding to a TPA cross-section of 54 GM. Furthermore, TP photolysis of 9 was found to induce the decomposition of the EGTA structure. This behaviour is superior to that of the NDBF derivatives, and makes it a promising TP-PPG, suggesting potential applications in physiology and neuroscience.
We thank N-BARD, Hiroshima University, for NMR and MS measurements. M. A. and K. K. gratefully acknowledge financial support from the Grant-in-Aid for Science Research on Innovative Areas “Stimuli-responsive Chemical Species” (No. 24109008 (MA); 25109544 (KK)) from the MEXT, Japan. C. K. acknowledges the HPC resources of CINES and of IDRIS under the allocations 2014-[x2014080649] made by GENCI (Grand Equipement National de Calcul Intensif).
Notes and references
- (a) J. Engels and E.-J. Schlaeger, J. Med. Chem., 1977, 20, 907–911 CrossRef CAS PubMed; (b) H. Kaplan, B. Forbush and J. F. Hoffmann, Biochemistry, 1978, 17, 1929–1935 CrossRef PubMed.
- (a) W. J. McEntee and T. H. Crook, Psychopharmacology, 1993, 111, 391–401 CrossRef CAS PubMed; (b) V. Parpura, T. A. Basarsky, F. Liu, K. Jeftinija, S. Jeftinija and P. G. Haydon, Nature, 1994, 369, 744–747 CrossRef CAS PubMed.
- (a) H. Mohler, Cell Tissue Res., 2006, 326, 505–516 CrossRef CAS PubMed; (b) P. Somogyi and T. Klausberger, J. Physiol., 2005, 562, 9–26 CrossRef CAS PubMed; (c) G. A. Ascoli, L. Alonso-Nanclares, S. A. Anderson, G. Barrionuevo, R. Benavides-Piccione, A. Burkhalter, G. Buzsaki, B. Cauli, J. DeFelipe, A. Fairen, D. Feldmeyer, G. Fishell, Y. Fregnac, T. F. Freund, D. Gardner, E. P. Gardner, J. H. Goldberg, M. Helmstaedter, S. Hestrin, F. Karube, Z. F. Kisvarday, B. Lambolez, D. A. Lewis, O. Marin, H. Markram, A. Munoz, A. Packer, C. C. H. Petersen, K. S. Rockland, J. Rossier, B. Rudy, P. Somogyi, J. F. Staiger, G. Tamas, A. M. Thomson, M. Toledo-Rodriguez, Y. Wang, D. C. West and R. Yuste, Nat. Rev. Neurosci., 2008, 9, 557–568 CrossRef CAS PubMed.
- (a) P. Klan, T. Solomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef CAS PubMed; (b) C. G. Bochet and A. Blanc, in Handbook of Synthetic Photochemistry, ed. A. Albini and M. Fagnoni, Wiley-VCH, Weinheim, 2010, pp. 417–447 Search PubMed.
- (a) C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed; (b) G. Bort, T. Gallavardin, D. Ogden and P. I. Dalko, Angew. Chem., Int. Ed., 2013, 52, 4526–4537 CrossRef CAS PubMed; (c) H. M. Kim and B. R. Cho, Chem. Rev., 2015, 115, 5014–5055 CrossRef CAS PubMed.
- (a) J. A. Barltrop and P. Schofield, Tetrahedron Lett., 1962, 3, 697–699 CrossRef; (b) D. H. R. Barton, Y. L. Chow, A. Cox and G. W. Kirby, Tetrahedron Lett., 1962, 3, 1055–1057 CrossRef; (c) J. C. Sheehan and R. M. Wilson, J. Am. Chem. Soc., 1964, 86, 5277–5281 CrossRef CAS; (d) J. A. Barltrop, P. J. Plant and P. Schofield, Chem. Commun., 1966, 822–823 RSC; (e) A. Patchornik, B. Amit and R. B. Woodward, J. Am. Chem. Soc., 1970, 92, 6333–6335 CrossRef CAS.
- (a) M. Matsuzaki, G. C. R. Ellis-Davis, T. Nemoto, Y. Miyashita, M. Iino and H. Kasai, Nat. Neurosci., 2001, 4, 1086–1092 CrossRef CAS PubMed; (b) K. Svoboda and R. Yasuda, Neuron, 2006, 50, 823–839 CrossRef CAS PubMed; (c) G. C. R. Ellis-Davies, Nat. Methods, 2007, 4, 619–628 CrossRef CAS PubMed; (d) A. Specht, F. Bolze, Z. Omran, J. F. Nicoud and M. Goeldner, HFSP J., 2009, 3, 255–264 CrossRef CAS PubMed; (e) M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 CrossRef CAS PubMed; (f) D. Warther, S. Gug, A. Specht, F. Bolze, J. F. Nicoud, A. Mourot and M. Goeldner, Bioorg. Med. Chem., 2010, 18, 7753–7758 CrossRef CAS PubMed; (g) G. C. R. Ellis-Davies, ACS Chem. Neurosci., 2011, 2, 185–197 CrossRef CAS PubMed; (h) J. P. Olson, H. B. Kwon, K. T. Takasaki, C. Y. Q. Chiu, M. J. Higley, B. L. Sabatini and G. C. R. Ellis-Davies, J. Am. Chem. Soc., 2013, 135, 5954–5957 CrossRef CAS PubMed; (i) J. M. Amatrudo, J. P. Olson, G. Lur, C. Q. Chiu, M. J. Higley and G. C. R. Ellis-Davies, ACS Chem. Neurosci., 2014, 5, 64–70 CrossRef CAS PubMed.
- TP uncaging efficiency (δu) is expressed by the multiplication of a TPA cross-section (σu in GM, 1 GM = 10−50 cm4 s per photon molecule) by the uncaging quantum yield ϕu, δu = σ2 × ϕu. OP uncaging efficiency, ε × ϕu, is expressed by the multiplication of an extinction coefficient ε in M−1 cm−1 by the uncaging quantum yield ϕu.
- Goeppert-Mayer (GM) unit: 1 GM = 10−50 cm4 s photon−1 molecule−1; named in honor of Maria Goeppert-Mayer, who set the theoretical basis for the TPA process, see: M. Goeppert-Mayer, Ann. Phys., 1931, 401, 273–294 CrossRef.
- N. I. Kiskin, R. Chillingworth, J. A. McCray, D. Piston and D. Ogden, Eur. Biophys. J., 2002, 30, 588–604 CrossRef CAS PubMed.
- (a) L. Donato, A. Mourot, C. M. Davenport, C. Herbivo, D. Warther, J. Leonard, F. Bolze, J. F. Nicoud, R. H. Kramer, M. Goeldner and A. Specht, Angew. Chem., Int. Ed., 2012, 51, 1840–1843 CrossRef CAS PubMed; (b) A. Specht, F. Bolze, L. Donato, C. Herbivo, S. Charon, D. Warther, S. Gug, J. F. Nicoud and M. Goeldner, Photochem. Photobiol. Sci., 2012, 11, 578–586 RSC; (c) C. Tran, T. Gallavardin, M. Petit, R. Slimi, H. Dhimane, M. Blanchard-Desce, F. C. Acher, D. Ogden and P. I. Dalko, Org. Lett., 2015, 17, 402–405 CrossRef CAS PubMed; (d) H. J. Yin, B. C. Zhang, H. Z. Yu, L. Zhu, Y. Feng, M. Z. Zhu, Q. X. Guo and X. M. Meng, J. Org. Chem., 2015, 80, 4306–4312 CrossRef CAS PubMed; (e) K. A. Korzycka, P. M. Bennett, E. J. Cueto-Diaz, G. Wicks, M. Drobizhev, M. Blanchard-Desce, A. Rebane and H. L. Anderson, Chem. Sci., 2015, 6, 2419–2426 RSC.
- A. Momotake, N. Lindegger, E. Niggli, R. J. Barsotti and G. C. R. Ellis-Davies, Nat. Methods, 2006, 3, 35–40 CrossRef CAS PubMed.
- The absorption maximum of NDBF was observed at 331 nm (ε = 18
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 945 M−1 cm−1) in DMSO, see the UV-vis spectrum of NDBF in the ESI† (Fig. S13a).
945 M−1 cm−1) in DMSO, see the UV-vis spectrum of NDBF in the ESI† (Fig. S13a). - (a) F. Terenziani, C. Katan, E. Badaeva, S. Tretiak and M. Blanchard-Desce, Adv. Mater., 2008, 20, 4641–4678 CrossRef CAS; (b) H. M. Kim and B. R. Cho, Chem. Commun., 2009, 153–164 Search PubMed; (c) S. Gug, F. Bolze, A. Specht, C. Bourgogne, M. Goeldner and J. F. Nicoud, Angew. Chem., Int. Ed., 2008, 47, 9525–9529 CrossRef CAS PubMed.
- G. S. He, L. S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245–1330 CrossRef CAS PubMed.
- S. Boinapally, B. Huang, M. Abe, C. Katan, J. Noguchi, S. Watanabe, H. Kasai, B. Xue and T. Kobayashi, J. Org. Chem., 2014, 79, 7822–7830 CrossRef CAS PubMed.
- M. Albota, D. Beljonne, J. L. Bredas, J. E. Ehrlich, J. Y. Fu, A. A. Heikal, S. E. Hess, T. Kogej, M. D. Levin, S. R. Marder, D. McCord-Maughon, J. W. Perry, H. Rockel, M. Rumi, C. Subramaniam, W. W. Webb, X. L. Wu and C. Xu, Science, 1998, 281, 1653–1656 CrossRef CAS PubMed.
- R. J. M. Anderson, G. R. Holtom and W. M. McClain, J. Chem. Phys., 1979, 70, 4310–4315 CrossRef CAS.
- The absorption maximum of NPBF was observed at 371 nm (ε = 23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 M−1 cm−1) nm in DMSO, see the UV-vis spectrum of NPBF in the ESI† (Fig. S13b).
260 M−1 cm−1) nm in DMSO, see the UV-vis spectrum of NPBF in the ESI† (Fig. S13b). - H. J. Lee, S. H. Kim, Y. R. Lee, X. Wang and W. S. Lyoo, Bull. Korean Chem. Soc., 2010, 31, 3027–3030 CrossRef CAS.
- A. K. Singh and P. K. Khade, Tetrahedron, 2005, 61, 10007–10012 CrossRef CAS.
- Photo-release of carboxylic acid derivatives, see: (a) B. Amit and A. Patchornik, Tetrahedron Lett., 1973, 14, 2205–2208 CrossRef; (b) B. Amit, D. A. Ben-Efraim and A. Patchornik, J. Am. Chem. Soc., 1976, 98, 843–844 CrossRef CAS; (c) J. Morrison, P. Wan, J. E. T. Corrie and G. Papageorgiou, Photochem. Photobiol. Sci., 2002, 1, 960–969 RSC; (d) A. D. Cohen, C. Helgen, C. G. Bochet and J. P. Tpscano, Org. Lett., 2005, 7, 2845–2848 CrossRef CAS PubMed; (e) M. Matsuzaki, T. Hayama, H. Kasai and G. C. Ellis-Davies, Nat. Chem. Biol., 2010, 6, 255–257 CrossRef CAS PubMed.
- M. Sheik-Bahae, A. A. Said, T. H. Wei, D. J. Hagan and E. W. Van Stryland, IEEE J. Quantum Electron., 1990, 26, 760–769 CrossRef CAS.
- G. C. R. Ellis-Davies, Chem. Rev., 2008, 108, 1603–1613 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, compound characterization and computational details. See DOI: 10.1039/c5cc07664a |
| This journal is © The Royal Society of Chemistry 2016 |