Open Access Article
Open Access ArticleReactivity differences of Sc3N@C2n (2n = 68 and 80). Synthesis of the first methanofullerene derivatives of Sc3N@D5h-C80†
Maira R.
Cerón‡
a,
Marta
Izquierdo‡
a,
Núria
Alegret
b,
Juan A.
Valdez
a,
Antonio
Rodríguez-Fortea
b,
Marilyn M.
Olmstead
*c,
Alan L.
Balch
*c,
Josep M.
Poblet
*b and
Luis
Echegoyen
*a
aDeparment of Chemistry, University of Texas at El Paso, El Paso, TX 79968, USA. E-mail: echegoyen@utep.edu; Tel: +1 915-747-7573
bDepartament de Química Física i Inorgànica, Universitat Rovira i Virgili, Tarragona 43007, Spain. E-mail: josepmaria.poblet@urv.cat; Tel: +34 977559569
cDepartment of Chemistry, University of California at Davis, Davis, CA 95616, USA. E-mail: mmolmstead@ucdavis.edu; albalch@ucdavis.edu; Tel: +1 530-752-0941
First published on 29th September 2015
Abstract
Using a purification method introduced earlier based on redox properties it was possible to isolate Sc3N@Ih-C80 and Sc3N@D3h-C78 and a mixture of Sc3N@D5h-C80 and Sc3N@D3-C68. Taking advantage of their chemical reactivity differences, Sc3N@D5h-C80 was isolated from Sc3N@D3-C68 followed by further functionalization, giving rise to five new methano-derivatives of Sc3N@D5h-C80.
In 1999 Stevenson et al. reported the third most abundant fullerene that can be prepared in an arc reactor, Sc3N@Ih-C80.1 The discovery of the trimetallic nitride endohedral fullerene (TNT) family includes the non-IPR Sc3N@D3-C68,2 the IPR Sc3N@D3h-C783 and the Sc3N@D5h-C80 isomer,4,5 all containing the same encapsulated triscandium nitride cluster inside.6–9
It is convenient that four different and interesting compounds are obtained in a single arcing experiment but their efficient separation presents many challenges. Several methods to isolate endohedral fullerenes have been reported,10–14 but none of them result in the efficient purification of Sc3N@D5h-C80. Taking advantage of the differences in the oxidation/reduction potentials of the members of the Sc3N@C2n (n = 34, 39, 40) family, we recently reported a selective chemical oxidation method that allows the isolation of pure Sc3N@Ih-C80 and Sc3N@C78, leaving a mixture of Sc3N@D5h-C80 and Sc3N@C68.15
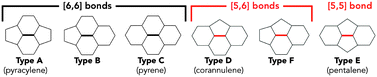
Exohedral functionalization of endohedral fullerenes is essential to improve their solubilities and to explore their properties and potential applications.16–20 There are very few examples of derivatives of Sc3N@C6821,22 and Sc3N@D5h-C80,4,5,23–26 partly because of their low synthetic yields and also due to the very large number of derivative isomers that can be obtained, which makes their purification and characterization very difficult. For example, the D3 symmetric non-IPR Sc3N@C68 possesses 18 different types of bonds (Fig. 1), so an unsymmetric addend could yield 35 possible regioisomers.7,9 In the case of the D5h symmetric Sc3N@C80, which has higher symmetry, there are nine different types of bonds and 15 possible regioisomer derivatives (Fig. 1).4,5
 | ||
| Fig. 1 Optimized structures for (a) Sc3N@D3-C68 and (b) Sc3N@D5h-C80 (Sc3N cluster is omitted for clarity). The (a) 18 and (b) 9 different types of bonds are labeled; [6,6]-junctions identified with numbers, [5,6] and [5,5]-junctions with letters. In the text and in Table 1, these bonds are labeled with the “b-” prefix to avoid confusion with the product isomers (1–8). | ||
There is only one example of an organic solar cell based on an endohedral fullerene acceptor (PCBH–Lu3N@Ih-C80).27 The main advantage of the endohedral fullerenes in photovoltaic applications results from their higher reduction potentials when compared to C60 and C70 (LUMO level),28 that results in higher open circuit voltages (Voc) and thus to higher power conversion efficiencies.
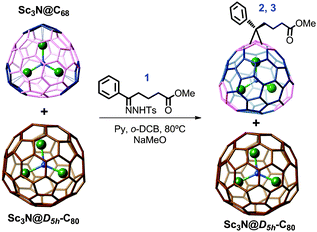
Here we report the separation of Sc3N@D5h-C80 from Sc3N@C68 based on their chemical reactivity differences.10,29,30 Exohedral functionalization of Sc3N@C68 was performed using a cyclopropanation reaction with the p-toluenesulfonyl tosyl hydrazone of phenyl butyric acid methyl ester to yield the corresponding PCBM-type derivatives. We also present for the first time the synthesis and characterization of five PCBM methano-derivatives of Sc3N@D5h-C80 (including the X-ray diffraction structure of one of the isomers) and two PCBM methano-derivatives of Sc3N@C68.
Separation of Sc3N@D5h-C80: a sample containing a mixture of Sc3N@D5h-C80 and Sc3N@C68 was obtained as described previously, by the selective oxidative/reductive removal of Sc3N@Ih-C80 and Sc3N@C78.15 Sc3N@D5h-C80 was then isolated from Sc3N@C68 by using a chemical reaction with the p-toluenesulfonyl tosyl hydrazone of phenyl butyric acid methyl ester (Compound 1, Scheme 1).31,32 This reaction afforded new endohedral derivatives of Sc3N@C68 and also provided a convenient separation method to purify Sc3N@D5h-C80.
The use of very accurate quantities of the reagents was crucial to successfully remove the Sc3N@C68 and isolate pure Sc3N@D5h-C80. Once the isomeric compounds 2 and 3 in Scheme 1 (PCBM–Sc3N@C68) are formed, the pristine endohedral fullerene (Sc3N@D5h-C80) can be easily separated from the reaction crude using regular silica gel column chromatography (Fig. S2, ESI†). To ensure the complete removal of Sc3N@C68, the reaction was followed by HPLC, taking an aliquot of the reaction crude and injecting it into the HPLC using a 5-PBB column. The optimized procedure employed 11.2 equivalents of tosyl hydrazone 1 per equivalent of Sc3N@C68 while heating during 1 hour at 95 °C. Using these conditions we recovered mainly multiple adducts of Sc3N@C68 as detected by MALDI-TOF (Fig. S3, ESI†) and a small fraction of monoadducts of PCBM–Sc3N@D5h-C80 (Fig. S3 and S4, ESI†).
PCBM–Sc3N@D3-C68: to characterize the PCBM–Sc3N@C68 monoadducts the reaction was repeated with fewer equivalents of 1 to yield two main monoadduct isomers (2 and 3, Fig. 2). The two isomers were purified by HPLC and characterized by 1H-NMR, mass spectrometry and UV-vis spectroscopy (Fig. S5–S11, ESI†). Although the D3 symmetry of Sc3N@C68 can result in 35 possible regioisomers, we only observed two, which suggests a highly regioselective formation pathway, possibly directed by the encapsulated cluster inside.33,34
 | ||
| Fig. 2 Recycling HPLC profile of the two isomers of PCBM–Sc3N@C68. Conditions: Bucky-clutcher column (φ = 250 mm × 10 ID); toluene; flow rate: 2 mL min−1; λ: 320 nm at room temperature. | ||
Sc3N@D3-C68 possesses eight different types of [6,6]-bonds, nine different types of [5,6]-bonds and one type of [5,5]-bond (Fig. 1a, Scheme 2). Due to the lack of symmetry of the addend used, with the spectroscopic evidences at hand, we cannot discard any of the 35 possible regioisomers for Sc3N@D3-C68.
Cyclopropanation reactions using hydrazones typically occur under thermodynamic control. Dorn and co-workers as well as Poblet and co-workers predicted that the main product under thermodynamic control for a Bingel reaction on Sc3N@D3-C68, is the “open” [6,6]-adduct on bond b-2 (Fig. 1a).21,34 All attempts to grow single crystals of these PCBM derivatives for structural assignment were unsuccessful; therefore DFT calculations were performed to determine the preferred addition sites. An adduct on bond b-2 has been also confirmed to be the lowest-energy product at 0 K as well as the most abundant isomer up to 353 K (80 °C) (see Table 1 and Fig. 1a, a full list of all the computed monoadducts can be found in the ESI†). Thus, if the reaction were to take place under thermodynamic control (vide infra), addition to bond b-2 would be the preferred product. The second preferred isomer would be on either bond b-a or bond b-b. Comparable results were observed by Akasaka and co-workers for a Diels–Alder reaction, where it was predicted by DFT that bonds adjacent to the pentalene unit are chemically more reactive.
| Reacting bondb | Bond type | E rel (kcal mol−1) |
|---|---|---|
| a Due to the non-symmetric addend, two different regioisomers are possible at each bond, with energy differences always within 1 kcal mol−1 (see ESI). For those bonds that contain a symmetry element (denoted with an asterisk) only one possible regioisomer exists. b Bonds are labelled without the prefix b- in Fig. 2. | ||
| Sc3N@D3-C68 | ||
| b-2* | [6,6] – Pyrene | 0.0 |
| b-a | [5,6] – Corannulene | 4.2 |
| b-b | [5,6] – Type F | 4.5 |
| b-e | [5,6] – Corannulene | 6.8 |
| b-3 | [6,6] – Type B | 8.4 |
| Sc3N@D5h-C80 | ||
| b-4* | [6,6] – Pyrene | 0.0 |
| b-c | [5,6] – Corannulene | 7.6 |
| b-b | [5,6] – Corannulene | 8.1 |
| b-a | [5,6] – Corannulene | 8.6 |
| b-1* | [6,6] – Type B | 8.7 |
| b-3 | [6,6] – Type B | 10.7 |
| b-2 | [6,6] – Type B | 11.2 |
| b-d | [5,6] – Corannulene | 11.6 |
| b-5* | [6,6] – Pyracylene | 22.1 |
PCBM–Sc3N@D5h-C80: the same PCBM reaction was performed using pure Sc3N@D5h-C80 and compared to that reported for the Ih-C80 isomer. While Sc3N@Ih-C80 yields only one PCBM derivative isomer, we observed five isomers for Sc3N@D5h-C80, out of the 15 distinct possibilities (4, 5, 6, 7 and 8).35 The five isomers were purified by HPLC (Fig. 3) and characterized by NMR spectroscopy, mass spectrometry and UV-vis spectroscopy (Fig. S12–S25). Cyclic voltammetry (CV) was recorded for isomers 4, 6, 7 and 8 and the crystal structure was obtained for isomer 7.
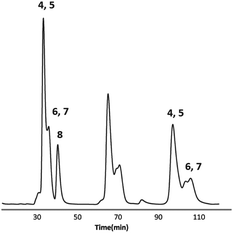 | ||
| Fig. 3 Recycling HPLC profile of the five isomers of PCBM–Sc3N@D5h-C80. Conditions: Bucky-clutcher column (φ = 250 mm × 10 ID); toluene; flow rate: 2 mL min−1; λ: 320 nm at room temperature. | ||
Sc3N@D5h-C80 possesses five different [6,6]-bonds and four different [5,6]-bonds (Fig. 1b, Scheme 2). Based on the available spectroscopic data it is not possible to discard any of the 15 possible regioisomers for Sc3N@D5h-C80.
Unfortunately, we cannot compare our results with the monoadduct of Sc3N@D5h-C80 synthetized via a 1,3-dipolar cycloaddition25,26 or to those calculated for the Diels–Alder additions,36–38 because it has been shown that for endohedral fullerenes different reaction types yield different regioisomers. For example, 1,3-dipolar cycloadditions on Sc3N@Ih-C80 result in addition to [5,6]-bonds as the thermodynamic product,39,40 while cyclopropanation reactions typically results in additions to [6,6]-bonds,17 with only one exception reported.41 For this reason we computed the lowest-energy regioisomers using DFT. The results collected in Table 1 show that bond b-4 leads to the thermodynamically preferred monoadduct, followed by bond b-c at 7.6 kcal mol−1. Adducts on bonds b-b, -a and -1 are between 8 and 9 kcal mol−1 higher in energy; the remaining adducts are at more than 10 kcal mol−1. Predicted molar fractions up to 400 K show that bond b-4 leads to the most abundant adduct (see ESI†). These results are in full agreement with the X-ray structural determination of compound 7, vide infra. Therefore, we might infer that this type of addition is likely to take place under thermodynamic control. If this were the case, adducts on bonds b-c and -b could be the other observed isomers in the HPLC chromatogram (compounds 4, 5, 6 and 8; Fig. 1b and 3). However, we cannot completely discard adducts on bonds b-a and -1, which are very close in energy to adducts on bonds b-c and -b. A detailed study of the reaction paths, is beyond the scope of this work.
Crystallographic results: black, single crystals of 7·CS2 were grown from a solution in CS2, CDCl3 and hexanes by slow diffusion. The structure of the compound is shown in Fig. 4. As the top drawing shows, the internal Sc3N group, which is ordered, is oriented so that one of the scandium ions is located near the site of addition. The lower drawing, which omits the Sc3N group for clarity, shows the position of addition along the band of hexagons at the middle of the D5h-C80 cage. Adduct formation has resulted in the rupture of the bond that formerly connected C38 and C43 in the cage. The non-bonded C38⋯C43 distance in 7 is 2.20(2) Å, whereas in pristine Sc3N@D5h-C80 the corresponding bonded C–C distance is 1.462 Å, which is the longest such distance in the cage.25
The redox properties were studied for isomers 4, 6, 7 and 8 of PCBM–Sc3N@D5h-C80 using CV, with a scan rate of 100 mV s−1 in o-DCB solutions and 0.05 M of n-Bu4NPF6 as supporting electrolyte. The CV results are summarized in Table S1, see ESI.† The cyclic voltammograms of isomers 4, 6, 7 and 8 showed three or four irreversible reduction waves, all cathodically shifted compared to those reported for the corresponding pristine fullerene Sc3N@D5h-C80.26 These results are in agreement with previous reports of cathodic shifts upon double bond removal by chemical functionalization.35,41 Isomers 4, 6, 7 and 8 also showed two or four reversible oxidation waves cathodically shifted compared to those of the corresponding pristine fullerene (Fig. S14, S19, S22 and S25, see ESI†).35,41
The CV results showed that PCBM methano-derivatives of the Sc3N@D5h-C80 possess higher reduction potentials when compared to PC61BM and PC71BM (LUMO level),31 thus higher open circuit voltages (Voc) could be expected when used in organic photovoltaic solar cells. The CV of isomers 7 and 8 exhibited very similar reduction potentials to those reported for PCBH–Lu3N@Ih-C80,27 thus if all other factors remain constant, similar open circuit voltages could be expected.
In conclusion, we report for the first time a convenient separation method of Sc3N@D5h-C80 from Sc3N@C68 based on chemical reactivity differences, and the exohedral functionalization of the two members of the Sc3N@C2n (n = 34 and 40) family, to yield the corresponding PCBM analogues. We also synthesized and characterized the first methano-derivatives of Sc3N@D5h-C80 and the X-ray diffraction structure of one of the isomers. Using Sc3N@C68 and the PCBM-diazo precursor we predict preferential addition on the b-2 bond, the same regioisomer reported for the 1,3-dipolar cycloaddition and the Bingel reaction.
L.E. thanks the NSF for generous support of this work under the grant (CHE-1408865) and to the NSF-PREM program (DMR-1205302). The Robert A. Welch Foundation is also gratefully acknowledged for an endowed chair to L.E. (AH-0033). A.L.B. and M.M.O. thank the U. S. NSF (CHE-1305125), and the Advanced Light Source, Beamline 11.3.1, Lawrence Berkeley Laboratory, for support. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract no. DE-AC02-05CH11231. J.M.P thanks MINECO (CTQ2014-52774-P) and the Generalitat of Catalonia (2014SGR-199 and XRQTC) for support.
Notes and references
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 402, 898 CrossRef CAS.
- S. Stevenson, P. W. Fowler, T. Heine, J. C. Duchamp, G. Rice, T. Glass, K. Harich, E. Hajdu, R. Bible and H. C. Dorn, Nature, 2000, 408, 427–428 CrossRef CAS PubMed.
- J. M. Campanera, C. Bo, M. M. Olmstead, A. L. Balch and J. M. Poblet, J. Phys. Chem. A, 2002, 106, 12356–12364 CrossRef CAS.
- J. C. Duchamp, A. Demortier, K. R. Fletcher, D. Dorn, E. B. Iezzi, T. Glass and H. C. Dorn, Chem. Phys. Lett., 2003, 375, 655–659 CrossRef CAS.
- M. Krause and L. Dunsch, ChemPhysChem, 2004, 5, 1445–1449 CrossRef CAS PubMed.
- K. Kobayashi, Y. Sano and S. Nagase, J. Comput. Chem., 2001, 22, 1353–1358 CrossRef CAS.
- M. M. Olmstead, H. M. Lee, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Angew. Chem., Int. Ed., 2003, 42, 900–903 CrossRef CAS PubMed.
- M. M. Olmstead, A. de Bettencourt-Dias, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Angew. Chem., Int. Ed., 2001, 40, 1223–1225 CrossRef CAS.
- S. S. Park, D. Liu and F. Hagelberg, J. Phys. Chem. A, 2005, 109, 8865–8873 CrossRef CAS PubMed.
- Z. Ge, J. C. Duchamp, T. Cai, H. W. Gibson and H. C. Dorn, J. Am. Chem. Soc., 2005, 127, 16292–16298 CrossRef CAS PubMed.
- C. D. Angeli, T. Cai, J. C. Duchamp, J. E. Reid, E. S. Singer, H. W. Gibson and H. C. Dorn, Chem. Mater., 2008, 20, 4993–4997 CrossRef CAS.
- S. Stevenson, K. Harich, H. Yu, R. R. Stephen, D. Heaps, C. Coumbe and J. P. Phillips, J. Am. Chem. Soc., 2006, 128, 8829–8835 CrossRef CAS PubMed.
- B. Elliott, L. Yu and L. Echegoyen, J. Am. Chem. Soc., 2005, 127, 10885–10888 CrossRef CAS PubMed.
- S. Stevenson, M. A. Mackey, J. E. Pickens, M. A. Stuart, B. S. Confait and J. P. Phillips, Inorg. Chem., 2009, 48, 11685–11690 CrossRef CAS PubMed.
- M. R. Cerón, F.-F. Li and L. Echegoyen, Chem. – Eur. J., 2013, 19, 7410–7415 CrossRef PubMed.
- T. Akasaka and S. Nagase, Endofullerenes: A New Family of Carbon Clusters, Dev. Fullerene Sci., Kluwer Academic Publishers, vol. 3, 2002 Search PubMed.
- M. Chen, X. Lu, M. R. Cerón, M. Izquierdo and L. Echegoyen, in Endohedral Metallofullerenes: Basic and Applications, ed. X. Lu, L. Echegoyen, A. L. Balch, S. Nagase and T. Akasaka, CRC Press, 2014, ch. 6, pp. 173–210 Search PubMed.
- M. N. Chaur, F. Melin, A. L. Ortiz and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 7514–7538 CrossRef CAS PubMed.
- D. M. Rivera-Nazario, J. R. Pinzón, S. Stevenson and L. A. Echegoyen, J. Phys. Org. Chem., 2013, 26, 194–205 CrossRef CAS.
- M. R. Cerón, F.-F. Li and L. A. Echegoyen, J. Phys. Org. Chem., 2014, 27, 258–264 CrossRef.
- T. Cai, L. Xu, C. Shu, J. E. Reid, H. W. Gibson and H. C. Dorn, J. Phys. Chem. C, 2008, 112, 19203–19208 CAS.
- T. Yang, X. Zhao, S. Nagase and T. Akasaka, Chem. – Asian J., 2014, 9, 2604–2611 CrossRef CAS PubMed.
- N. B. Shustova, A. A. Popov, M. A. Mackey, C. E. Coumbe, J. P. Phillips, S. Stevenson, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2007, 129, 11676–11677 CrossRef CAS PubMed.
- S. Yang, C. Chen, M. Jiao, N. B. Tamm, M. A. Lanskikh, E. Kemnitz and S. I. Troyanov, Inorg. Chem., 2011, 50, 3766–3771 CrossRef CAS PubMed.
- S. Osuna, A. Rodriguez-Fortea, J. M. Poblet, M. Sola and M. Swart, Chem. Commun., 2012, 48, 2486–2488 RSC.
- T. Cai, L. Xu, M. R. Anderson, Z. Ge, T. Zuo, X. Wang, M. M. Olmstead, A. L. Balch, H. W. Gibson and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 8581–8589 CrossRef CAS PubMed.
- R. B. Ross, C. M. Cardona, D. M. Guldi, S. G. Sankaranarayanan, M. O. Reese, N. Kopidakis, J. Peet, B. Walker, G. C. Bazan, E. Van Keuren, B. C. Holloway and M. Drees, Nat. Mater., 2009, 8, 208–212 CrossRef CAS PubMed.
- C. M. Cardona, B. Elliott and L. Echegoyen, J. Am. Chem. Soc., 2006, 128, 6480–6485 CrossRef CAS PubMed.
- H. Shinohara, Rep. Prog. Phys., 2000, 63, 843–892 CrossRef CAS.
- L. Dunsch and S. Yang, Small, 2007, 3, 1298–1320 CrossRef CAS PubMed.
- J. C. Hummelen, B. W. Knight, F. LePeq, F. Wudl, J. Yao and C. L. Wilkins, J. Org. Chem., 1995, 60, 532–538 CrossRef CAS.
- B. Q. Mercado, M. N. Chaur, L. Echegoyen, J. A. Gharamaleki, M. M. Olmstead and A. L. Balch, Polyhedron, 2013, 58, 129–133 CrossRef CAS.
- A. A. Popov and L. Dunsch, J. Am. Chem. Soc., 2007, 129, 11835–11849 CrossRef CAS PubMed.
- N. Alegret, A. Rodríguez-Fortea and J. M. Poblet, Chem. – Eur. J., 2013, 19, 5061–5069 CrossRef CAS PubMed.
- C. Shu, W. Xu, C. Slebodnick, H. Champion, W. Fu, J. E. Reid, H. Azurmendi, C. Wang, K. Harich, H. C. Dorn and H. W. Gibson, Org. Lett., 2009, 11, 1753–1756 CrossRef CAS PubMed.
- S. Osuna, R. Valencia, A. Rodríguez-Fortea, M. Swart, M. Solà and J. M. Poblet, Chem. – Eur. J., 2012, 18, 8944–8956 CrossRef CAS PubMed.
- S. Osuna, M. Swart, J. M. Campanera, J. M. Poblet and M. Solà, J. Am. Chem. Soc., 2008, 130, 6206–6214 CrossRef CAS PubMed.
- T. Yang, S. Nagase, T. Akasaka, J. M. Poblet, K. N. Houk, M. Ehara and X. Zhao, J. Am. Chem. Soc., 2015, 137, 6820–6828 CrossRef CAS PubMed.
- C. M. Cardona, A. Kitaygorodskiy, A. Ortiz, M. A. Herranz and L. Echegoyen, J. Org. Chem., 2005, 70, 5092–5097 CrossRef CAS PubMed.
- T. Cai, Z. Ge, E. B. Iezzi, T. E. Glass, K. Harich, H. W. Gibson and H. C. Dorn, Chem. Commun., 2005, 3594–3596 RSC.
- M. Izquierdo, M. R. Cerón, M. M. Olmstead, A. L. Balch and L. Echegoyen, Angew. Chem., Int. Ed., 2013, 52, 11826–11830 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1421222. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc07416a |
| ‡ M.R.C. and M.I. contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |