Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biomaterials-based strategies for salivary gland tissue regeneration
Tugba
Ozdemir
a,
Eric W.
Fowler
a,
Ying
Hao
a,
Anitha
Ravikrishnan
a,
Daniel A.
Harrington
b,
Robert L.
Witt
cd,
Mary C.
Farach-Carson
bc,
Swati
Pradhan-Bhatt
*cde and
Xinqiao
Jia
*ace
aDepartment of Materials Science and Engineering, University of Delaware, Newark, DE 19716, USA
bDepartments of BioSciences and Bioengineering, Rice University, Houston, TX 77251, USA
cDepartment of Biological Sciences, University of Delaware, Newark, DE 19716, USA
dHelen F. Graham Cancer Center and Research Institute, Christiana Care Health Systems, Newark, DE 19713, USA
eDepartment of Biomedical Engineering, University of Delaware, Newark, DE 19716, USA. E-mail: swati@udel.edu; xjia@udel.edu; Fax: +302-623-4314, +302-831-4545; Tel: +302-623-4649, +302-831-6553
First published on 15th February 2016
Abstract
The salivary gland is a complex, secretory tissue that produces saliva and maintains oral homeostasis. Radiation induced salivary gland atrophy, manifested as “dry mouth” or xerostomia, poses a significant clinical challenge. Tissue engineering recently has emerged as an alternative, long-term treatment strategy for xerostomia. In this review, we summarize recent efforts towards the development of functional and implantable salivary glands utilizing designed polymeric substrates or synthetic matrices/scaffolds. Although the in vitro engineering of a complex implantable salivary gland is technically challenging, opportunities exist for multidisciplinary teams to assemble implantable and secretory tissue modules by combining stem/progenitor cells found in the adult glands with biomimetic and cell-instructive materials.
1. Introduction
Salivary glands, including the parotid, submandibular, and sublingual glands as well as numerous minor glands, produce saliva in response to a wide range of biochemical input and environmental cues. Control of response is achieved through the cooperative actions of various cell types that are organized into a complex branched acinar and ductal structure.1,2 Located in the upper aerodigestive tract, the salivary gland can be damaged by radiation therapy for head and neck cancers, and the patients’ quality of life can be severely compromised owing to the reduced saliva production and altered saliva composition. Manifested as “dry mouth syndrome”, or xerostomia, patients suffer from oral dryness, have difficulty speaking, swallowing, and can develop dental caries and periodontal diseases. Current treatments for xerostomia temporarily mitigate the symptoms, but do not provide long-term therapeutic benefits.3In 2000, Baum and colleagues proposed the concept of producing an artificial, tissue-engineered salivary gland as a potential clinical solution for xerostomia. Their initial report highlighted the importance of presenting appropriate matrix proteins on porous polyester scaffolds for the attachment and growth of a human salivary gland-derived cell line.4 Our collaborative team further refined the conditions and procedures for salivary gland tissue engineering using primary human salivary gland epithelial cells isolated from patients undergoing head and neck surgery pre-radiation. These isolated cells are cultured in vitro in synthetic matrices to stimulate cellular organization and assembly into complex three-dimensional (3D) structures. Ultimately, the engineered construct containing integrated structural components will be implanted at the site of radiation injury for tissue regeneration purposes.5 Such an autologous cell-based, reverse engineering approach for salivary gland restoration is challenging, as the tissue development and maturation depends on the reciprocal interactions between various types of cells and tissues comprising and surrounding the gland to promote cell survival, proliferation, apoptosis, adhesion, motility and morphological changes. Ex vivo culture of mouse embryonic submandibular gland tissues has revealed key insights in developmental biology,6–8 that can inform and direct biomaterials-based approaches for reconstitution of salivary gland architecture and function. The inaccessibility of human embryonic tissues/cells and their tumorigenic transformation upon implantation limit their usage in salivary gland tissue engineering.9 Therapeutic salivary gland regeneration is possible if adult stem/progenitor cells can be harvested and reprogrammed to maximize their regenerative capacity.6
Although cell–cell interactions dictate the assembly of epithelial tissues, the extracellular matrix (ECM), whether in the form of the basement membrane in direct contact with the structural units or as a 3D mesenchyme surrounding the organized salivary gland tissue, provides biophysical, biomechanical and biochemical cues to guide the epithelial cells into organized structures and functional tissues.10,11 Biomaterials designed for tissue engineering applications must be biocompatible, biodegradable, biologically relevant and exhibit tissue-like viscoelasticity. For the ex vivo culture of cells of epithelial origin, one must consider the potential of synthetic matrices to foster cell–cell contact/communication, guide cellular assembly, direct polarization and induce branching.12
In this mini review, we first outline the basic structure of the human salivary gland. We then provide examples of biologically inspired material designs applied to salivary gland tissue engineering. These are presented in the context of 2D and 3D culture of adult salivary gland cells, as well as ex vivo culture of embryonic tissues. We discuss how properties of materials affect cellular functions and how materials-derived models can be exploited to gain understanding of tissue morphogenesis. Salivary gland tissue engineering is still in infancy and many technical and regulatory challenges remain before an implantable tissue analog can be translated into the clinic. Nevertheless, it is our belief that the design of tunable, dynamic and cell-instructive matrices with environmental cues and ECM-derived motifs will ultimately lead to the development of a deliverable implant device for patients suffering from xerostomia.
2. Salivary gland anatomy and physiology
The salivary gland achieves its secretory function via the coordinated actions of assembled acinar, ductal, and myoepithelial cells (Fig. 1). While the acinar cells form functional spherical acini with lumen into which they secrete proteins and fluid, the ductal cells create a tubular conduit to transport acini-derived saliva with slight modifications, into the oral cavity. As the protein-rich salivary mixture flows through the ductal network, its ionic composition is modified. In their respective units, the acinar and the ductal cells are linked together by complementary cell junctions, such as occludin, anchoring and communicating junctions. The cytoskeletal filaments, along with attached cell–cell and cell–matrix adhesion sites, maintain the structural and mechanical integrity of the assemblies. The organelles and membrane proteins in these cells segregate heterogeneously in various locations of the intracellular space such that apical, lateral and basal surfaces form.1,5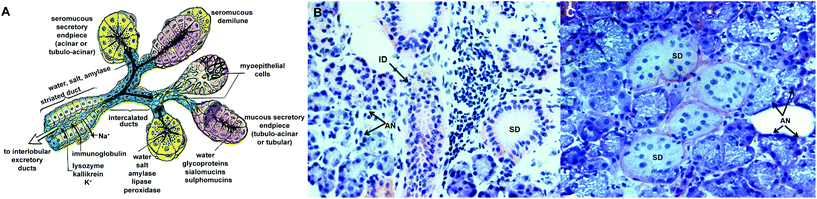 | ||
Fig. 1 Structure and organization of the human salivary gland. (A) Schematic illustration of the cross-sectional view of the salivary gland composed of the serous acinus and the intercalated duct (adapted from Gray et al., 1995![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 with permission). (B) Hematoxylin and eosin staining of human salivary gland tissue (20×). (C) Periodic acid Schiff staining of the salivary gland tissue (40×). Arrows point to AN, acini; ID, intercalated duct; and SD, striated ducts. 93 with permission). (B) Hematoxylin and eosin staining of human salivary gland tissue (20×). (C) Periodic acid Schiff staining of the salivary gland tissue (40×). Arrows point to AN, acini; ID, intercalated duct; and SD, striated ducts. | ||
The epithelial layer, whether in the acinus or in the duct, overlies a basement membrane that is ∼100 nm thick, mainly composed of collagen IV, laminin, nidogen and the proteoglycan perlecan/HSPG2. Also present in the basement membrane are proteases and their inhibitors, as well as growth and regulatory proteins, many being sequestered by the heparan sulfate chains of perlecan. The epithelial cells are attached to the basement membrane through integrin heterodimers located at the basal membrane of the cells. In a polarized epithelial cell, the basal membrane contains neurotransmitter receptors and some ion channels, while the junctional complexes containing E-cadherin and zonula occludens are found near the apex of the lateral membrane. The apical membrane contains aquaporins and mucins. Myoepithelial cells wrap around the acini inside the basement membrane that separates them from the surrounding stroma, purportedly expelling primary saliva from the acini through actomyosin-mediated contraction.13 The tight regulation of ECM composition and cell–cell interactions maintain a polarized structure with a directional secretory function.10
During development, the salivary gland epithelium undergoes programmed expansion and morphogenesis to form a complex tissue architecture with branched, interconnected and well-ordered lobules and ducts. Such morphological transformation requires intimate epithelial–mesenchymal crosstalk via frequent cycles of cleft formation and bud outgrowth, effectively maximizing surface area needed to provide sufficient trans-epithelial fluid secretion. The complex glandular structures bounded by the basement membrane are surrounded by stromal tissues and are innervated by the peripheral nervous system, which controls saliva production through sympathetic and parasympathetic mechanisms.11,14
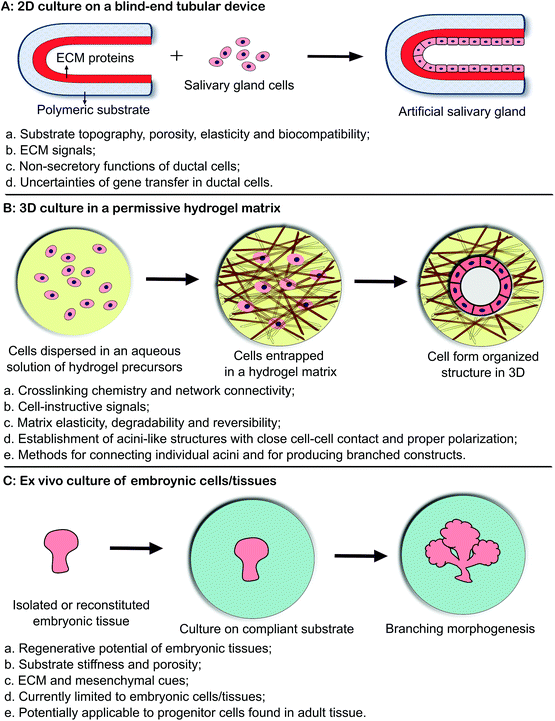
3. Biomaterials strategy
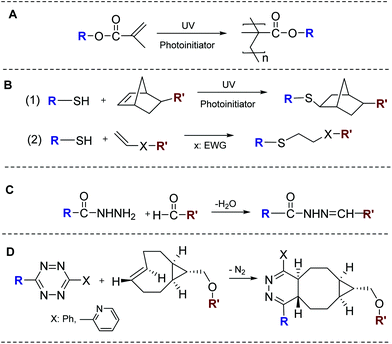
Biomaterials-based tissue engineering strategies for the restoration of salivary gland function can be generally divided into three categories (Fig. 2 and Table 1). In the first approach (2D culture, Fig. 2A),4 primary salivary gland cells or cell lines are introduced and cultured as a monolayer lining a blind-end tubular device of porous and biodegradable polymers. The goal is to create a polarized epithelial cell monolayer capable of unidirectional fluid secretion. Other 2D culture studies aim at promoting acinar cell phenotype, expanding the desired cell population or generating 3D aggregates on 2D surfaces. In the second approach (3D culture, Fig. 2B),15 selected stem/progenitor and epithelial cell populations from dispersed salivary gland cells are encapsulated in 3D hydrogel matrices, frequently constructed employing bioorthogonal chemistries (Fig. 3), and allowed to proliferate and assemble. 3D assembly can reconstitute the polarized and secretory acinar structures that are envisioned to connect and integrate with the existing ductal structure in the tissue once implanted post radiation. Native cellular replacement strategies rely on the availability of expanded progenitor cell populations from adult human tissues with inherent assembly capacity, secretory functions and regenerative potential. In the third approach (ex vivo culture of embryonic tissues, Fig. 2C),16 embryonic salivary gland tissues or cells are cultured on a compliant substrate to allow for branching morphogenesis to occur in vitro. These studies have revealed key insights into the developmental biology of the salivary gland, providing guidance in the design of effective therapies for the repair of damaged glands and the regeneration of functional substitutes.| Approach | Biomaterials | Cells/tissues | Major observation |
|---|---|---|---|
| 2D culture | PLA and PGA (flat disks) | Immortalized human salivary gland cell line (HSG) | Coating of matrix proteins is necessary to support cell attachment and organization.4,92 |
| PLGA (fibrous scaffolds) | Immortalized adult mouse submandibular gland ductal cell line (SIMS) | More rounded and clustered cell morphology as compared to those grown on planner surfaces; polarization and the establishment of tight junctions require laminin coating; additional physical features of the substrate, such as curvature, affects cell polarization and expression of tight junction and water channel proteins.26–28 | |
| Immortalized adult rat parotid gland acinar cell line (ParC10) | |||
| Silk fibroin (porous scaffolds) | Primary salivary gland epithelial cells from rat submandibular gland and parotid gland | Promote epithelial cell growth, facilitate the secretion of matrix proteins and retain the differentiated function.20 | |
| HA hydrogels | Primary human salivary gland acinar-like cells from the parotid gland | Acini-like structures with tight junctions, α-amylase expression and an apoptotic central lumen was observed on HA gels with an elastic modulus of 2000 Pa and incorporating PlnDIV peptide.23 | |
| [PEG(RGD)-C12]n | Human primary salivary gland myoepithelial cells | Provide guidance cues for the attachment and elongation of myoepithelial cells.31 | |
| 3D culture | PEG hydrogels | A mixture of primary acinar and ductal cells from mouse submandibular gland | Cells survive the encapsulation in the thiol–ene network, but remain as single cells without forming organized acini-like structures; encapsulation of pre-assembled spheroids improved viability, promoted cell proliferation, and established and preserved cell–cell contacts.41 |
| HA/PEG hydrogels | Primary human salivary gland acinar-like cells from the parotid gland | Cells self-assembled into acini-like structures ∼50 μm in size; the structures demonstrated neurotransmitter-stimulated protein secretion and fluid production; incorporation of PlnDIV peptide in the hydrogel induced lumen formation.15,23,37 | |
| Ex vivo culture of embryonic tissues | PLGA fibrous scaffold; PVDF or chitosan membrane | Mouse embryonic submandibular glands | Support the branching morphogenesis if embryonic salivary gland16,69 |
| Alginate or polyacrylamide gels | Mouse embryonic submandibular glands | Surface immobilization of cell adhesive peptide or protein is necessary; softer gels enhance the bud expansion and cleft formation, whereas stiffer gels attenuate them; partial rescue of acini structure and differentiation can be achieved by adding exogenous growth factors or by transferring glands from stiff to soft substrates. |
3.1. 2D culture
The original design of an artificial salivary gland17 requires a secretory device containing a cohesive monolayer of ductal cells lining the interior of a tube fabricated from commercial polymers that not only promote cell attachment and growth, but also preserve the desired cell phenotype. Yamada and Baum investigated the suitability of biodegradable polyesters (PLA: poly(lactic acid), PGA: poly(glycolic acid)) for the growth and organization of a human salivary ductal epithelial cell line (HSG). In general, substrates without any ECM coating do not support robust cell attachment and growth. Substrates coated with proteins derived from basement membrane (Matrigel®, laminin, or collagen IV) foster the slow growth of adherent cells and facilitate the development of organized 2D cell aggregates. Substrates coated with proteins more characteristic of interstitial tissue (collagen I or fibronectin) promote the rapid development of HSG cell monolayers.4 However, the inability of HSG cells to form a polarized monolayer and to establish tight junctions, combined with their potential to undergo malignant transformation in vivo, prohibited the widespread usage of these cells for salivary gland tissue engineering purposes.9,18As researchers continue to identify and isolate human cells for salivary gland tissue engineering, parallel effort has been focused on the development of appropriate biomaterial scaffolds to support the proliferation and differentiation of salivary gland progenitors. Porous membranes or scaffolds of relatively hydrophobic polymers, such as poly(vinylidene fluoride) (PVDF) or silk fibroin, are more supportive of cell growth19 and phenotype retention20 than flat substrates. Fibronectin-coated silk fibroin scaffolds supported the development of aggregates that resemble the secretory acini morphologically and functionally. Cells cultured under these conditions maintained their differentiated secretory function for approximately one month. These materials can be used for growing and expanding highly differentiated salivary gland cells for regeneration purposes. Therefore, a tubular scaffold with dense outer surface to prevent saliva leakage and a porous inner surface for the cell attachment and growth can be utilized for the creation of an artificial salivary gland.
Under appropriate conditions, monolayer culture on flat surfaces also can give rise to multicellular spherical structures, expressing acinar-like phenotype. Our group conducted a pilot study by culturing primary human salivary gland cells on photocrosslinked hyaluronic acid (HA) hydrogels incorporating an 18-amino acid long peptide, identified from the domain IV of perlecan (PlnDIV) and known to support cell adhesion, spreading and FAK activation.21,22 Self-assembly of acini-like structures with tight junctions, α-amylase expression and an apoptotic central lumen was observed among structures formed on these HA-based gels.23 Separately, primary human parotid gland acinar cells spontaneously formed 3D cell aggregations after reaching confluence on tissue culture polystyrene (TCPS), and more frequently, on poly (lactic-co-glycolic acid) (PLGA). However, these post-confluence 3D structures are fairly disorganized, potentially because of the absence of basement membrane signals.17
In a comparative study, human parotid and submandibular gland cells were plated onto either Matrigel®-coated or uncoated TCPS. On uncoated plastic surfaces, monolayers of ductal cells with tight junctions were observed. On Matrigel®-coated substrates, cells formed 3D acinar-like units, adopted an acinar phenotype with many secretory granules, and expressed α-amylase and the water channel protein, aquaporin-5 (AQP5).24 Work from our group shows that coating of TCPS with PlnDIV peptide or Matrigel® elicited the same cellular responses from primary human salivary gland cells, and both coatings support the formation of 3D acini-like salivary units that express α-amylase. The synthetic nature of PlnDIV peptide enables the culture of human acinar cells free of animal products, thus representing a step forward towards the creation of implantable artificial gland.22
Owing to the structural similarities to the basement membrane, fibrous polymer scaffolds,25 most often produced by electrospinning, have been used to culture salivary gland epithelial cells. In an exploratory study, Larsen and co-workers26 investigated the effects of topography on behaviors of immortalized adult mouse or rat salivary gland cell lines (SIMS, ductal; Par-C10, acinar). Compared to cells grown on planar surfaces of the same composition, cells cultured on the fibrous scaffolds exhibited a more rounded and clustered morphology, as well as a reduced and more diffuse expression of focal adhesion proteins. A follow-up study27 revealed that cell proliferation and polarization strongly depend on the surface coating of the nanofiber scaffolds. While chitosan coating promoted cell proliferation, appropriate polarization and mature tight junctions were observed only when the scaffold was coated with laminin-111. Bifunctional scaffolds containing chitosan and laminin-111 signals induced responses from both acinar and ductal cell lines.
To further mimic the architecture of the basement membrane surrounding spherical acini of salivary gland epithelial cells, Soscia28 created ordered arrays of “craters” in polydimethylsiloxane (PDMS) and lined them with electrospun poly(lactic acid-co-glycolic acid) (PLGA) nanofibers (Fig. 4E). Using SIMS and Par-C10 cells, the authors found that increasing crater curvature increased the average height of the SIMS cell monolayer, cell polarization, cellular expression (both SIMS and Par-C10 cells) of AQP5 and tight junction protein occludin in Par-C10 cells, This work highlights the potential of physical features, including surface chemistry and scaffold stiffness, to promote differentiation of salivary gland cells.
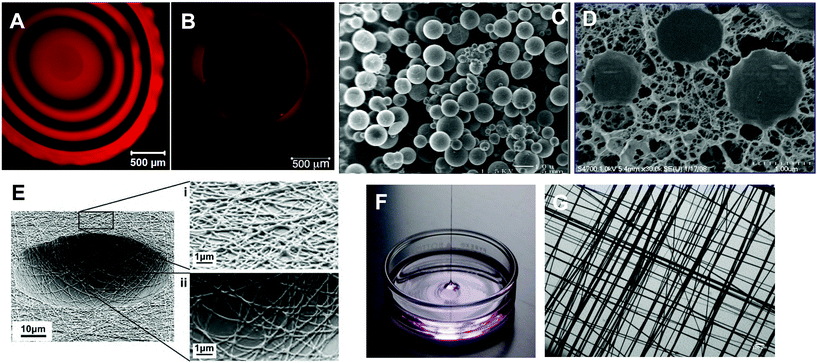 | ||
| Fig. 4 Representative microscopy images of biomaterials developed for salivary gland tissue engineering. (A) Confocal microscopy image of the central slice of a HA-based solid hydrogel sphere spatially tagged TCO-modified Alexa Fluor® 647 (red). Black regions correspond to crosslinked HA gel layer without the dye. (B) A z-stack confocal image showing the top view of the HA hydrogel channel covalently labeled with Alexa Fluor® 647 (red). Black region inside the red wall corresponds to a water-filled channel interior. (C) Scanning electron micrograph (SEM) of HA-based HGPs. (D) Cryogenic SEM image of HA-based doubly crosslinked networks. (E) SEM image of PLGA nanofibrous crater created by electrospinning and photolithography. (F) Digital picture showing a multiblock copolymer fiber pulled out of the oil/water interface during the interfacial bioorthogonal polymerization process. (G) Crosshatched multiblock copolymer mesh imaged under light microscope. Reprinted with permission from Zhang et al., 2014 (A and B),51 Jia et al., 2006 (C),52 Jha et al., 2009 (D),56 Soscia et al., 2013 (E)28 and Liu et al., 2015 (F and G).31 | ||
Although processing polymers into fibrous scaffolds by electrospinning is straightforward, the direct incorporation of biological motifs during electrospinning is more complicated. Moreover, the physical and mechanical properties of the fibrous scaffolds cannot be tuned easily using the same polymer. Work from the Fox and Jia laboratories has demonstrated the utility of tetrazine (Tz) ligation with trans-cyclooctenes (TCO), a highly efficient, bioorthogonal reaction29,30 (Fig. 3D) that proceeds with exceptional rates without any catalysis, for the de novo synthesis of multiblock copolymer fibers (Fig. 4F and G).31 Using a hydrophilic poly(ethylene glycol) (PEG)-based tetrazine monomer and an aliphatic TCO monomer with a dodecyl (C12) linker, the polymerization can be carried out at the immiscible water/oil interface. As the polymerization proceeded, mechanically robust polymer fibers (9–10 μm in diameter) with molecular weight up to 263 kDa were continuously pulled out of the interface. The bioorthogonal nature of the tetrazine ligation permits facile incorporation of functional peptides into the multiblock constructs. When a fibronectin-based peptidic building block (GRGDSP) was included in the monomer mixture, interfacial bioorthogonal polymerization produced mechanically robust cell-adhesive microfibers. Human salivary gland myoepithelial cells attached to the RGD fibers, developed long and narrow lamellipodia and oriented parallel to the long axis of the fiber. In some cases, multiple cells formed a cohesive blanket enclosing the fiber.31 Overall, the peptide-containing fibers present appropriate biochemical signals and topographical features for the anchorage and alignment of myoepithelial-like cells that may facilitate assembly of fully functional salivary gland tissues.
3.2. 3D culture
Isolated salivary gland cells traditionally are cultured in hydrogels derived from natural proteins extracted from animal tissues, such as Matrigel®,32 collagen gel,33 and fibrin gel,34 or a mixture of fibrin gel and collagen gel.35 In these hydrogel systems, dispersed salivary gland cells divide and assemble into 3D acinar-like and/or ductal-like structures, where, depending on phenotype, they express subsets of tight junction proteins, such as ZO-1, occludin, and claudin-1, and a critical water channel protein, AQP5. Additional growth and differentiation factors are necessary to create and maintain the differentiated phenotype, to stabilize the basic functional units and to induce branching.32–34 To more fully recapitulate the native salivary gland microenvironment, decellularized submandibular glands were used as scaffolds for the 3D culture of rat submandibular gland cells. Cells seeded into the scaffold via injection through the main gland duct and cultured under rotational conditions, adhered to the scaffold, expressed the differentiated markers, and formed gland-like tissues.36While generally conducive to cell assembly, migration and organization, reconstituted biomaterials derived from natural tissues lack the tunability and reproducibility seen in synthetic matrices and are potentially tumorigenic or immunogenic. Thus, there is a critical need to develop synthetic matrices or scaffolds that recreate the developmental niches and exhibit tunable properties and cell-instructive signals for the establishment of functional and clinically translatable products to relieve xerostomia. To date, synthetic hydrogels utilized for salivary gland tissue engineering purposes are based largely on PEG and HA.15,37,38 Synthesized by living ring-opening polymerization,39 mono-disperse PEG with controlled molecular weight and defined end groups are commercially available.40 Shubin et al. evaluated the suitability of PEG-based hydrogels, crosslinked by radical chain polymerization (Fig. 3A) or thiol–ene polymerization (Fig. 3B1), for the 3D culture of primary mouse submandibular gland (SMG) cells, a mixture of acinar and ductal cells. Although the thiol–ene network was found to be more cytocompatible than the radically crosslinked counterpart, the SMG cells entrapped at single cell state in both types of gels failed to form organized structures. Encapsulation of pre-assembled multicellular spheroids improved cell viability, promoted cell proliferation, and established and preserved cell–cell contacts.41
Although not present in the basement membrane of the epithelium, HA is a ubiquitous, non-sulfated glycosaminoglycan (GAG) found in the surrounding mesenchyme, and is especially abundant in early embryos. Unlike PEG, HA is biologically active, binding specific cell surface receptors and directing multiple cell functions including adhesion, migration, and morphogenesis.42 High molecular weight (1–2 MDa) HA is produced by bacterial fermentation. Subsequent degradation of the high molecular weight HA by chemical or enzymatic means results in medium or low molecular weight fragments.38 Our group has synthesized HA derivatives bearing mutually reactive functional groups that participate in Michael addition (Fig. 3B2)43 or hydrazone ligation (Fig. 3C)44,45 to initiate fast, biocompatible gelation in bulk for the fabrication of cell-laden gel constructs.46,47
Our collaborative team is investigating the utility of these synthetic matrices for salivary gland tissue engineering purposes. Pradhan et al. developed methods for isolating salivary gland acinar-like cell populations from tissue specimens harvested from patients undergoing head and neck surgery.22 Primary human salivary gland cells were encapsulated in an HA hydrogel with an elastic modulus of 60–100 Pa.15 Overtime, cells self-assembled into organized acini-like structures ∼50 μm in size (Fig. 5). Additionally, neurotransmitter stimulation of these acini-like structures via β-adrenergic agonists led to increased granule and α-amylase production. Cholinergic stimulation led to intracellular calcium release with oscillations within these structures, indicative of an active fluid production pathway. Encapsulated cells in 3D retained their spheroid structure and structural integrity, along with the salivary biomarkers and maintained viability for over three weeks in vivo in an athymic rat model.37 As discussed above, the inclusion of PlnDIV peptide in 2D cultures on HA gels stimulates the formation of polarized acinus with a hollow lumen.23 We have synthesized macromolecular version of PlnDIV (MacroPlnDIV) by adopting our established polymerization and conjugation protocols48–50 to present multiple repeats of the peptide signals along the polymer backbone similar to those in native perlecan domain IV. Our ongoing effort is dedicated to the incorporation of MacroPlnDIV in HA hydrogels to elicit the desired cell assembly/polarization via potent and coordinated cell–matrix interactions.
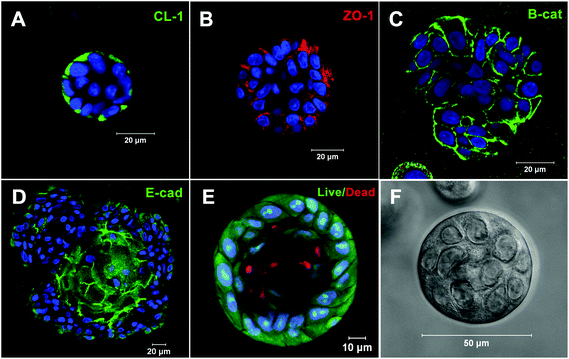 | ||
| Fig. 5 Acini-like spheroids in 3D HA hydrogels. Spheroid structures express tight junction markers (A) CL-1, (B) ZO-1, (D) E-cadherin and adherens junction marker, (C) β-catenin. (E) Live/dead staining of Syto13 positive green cells and propidium iodide positive red cells. Nuclei are stained in blue. (F) Representative phase image of an acinus-like structure. Reprinted from Pradhan-Bhatt et al., 201315 with permission. | ||
Recently, tetrazine ligation (Fig. 3D) has been applied to hydrogel synthesis via an interfacial bioorthogonal gelation process using high molecular weight tetrazine-modified HA (HA-Tz, 218 kDa) and low molecular weight PEG-based TCO crosslinker (bisTCO, 1253 Da). Because the crosslinking is diffusion controlled, hydrogel spheres with 3D spatial patterns (Fig. 4A) and water-filled hydrogel channels (Fig. 4B) can be fabricated readily without the need for external templates or stimuli.51 These bioorthogonal hydrogel platforms are being explored for the in vitro assembly of secretory acini with interconnected ducts.
In addition to immobilized peptide signals, soluble growth factors presented in the hydrogel matrix in a spatio/temporal manner are indispensable to generate interconnected and branched salivary gland structures. Other growth factors initiate innervation and angiogenesis needed for host integration. We have synthesized stably crosslinked, nanoporous HA-based hydrogel particles (HGPs, Fig. 4C) by inverse emulsion polymerization.52,53 HGPs decorated with perlecan domain I (PlnDI)54 or heparin55 sequester heparin binding growth factors and control their release. Doubly crosslinked networks (Fig. 4D) have been created using HA HGPs as the structural units, cell attachment points and growth factor depots to promote desired cellular responses necessary for the regeneration of functional neotissues.43,56–58
3.3. Ex vivo culture
The third strategy for salivary gland regeneration relies on the intrinsic power of embryonic tissues to undergo programmed branching morphogenesis for the creation of replacement tissue by ex vivo culture of embryonic salivary glands.59 Further, ex vivo organ culture using mouse embryonic glands has shed critical insight on salivary gland development.8,11,14 Key features of the expanding embryonic tissue include: (1) a distensible basement membrane that undergoes dynamic remodeling by proteolytic degradation;60 (2) an enhanced motility of the outer epithelial bud cells mediated through integrin-dependent cell–matrix association;61 (3) a mechanochemical checkpoint for cleft initiation/progression;62 and (4) a deposition of fibronectin in the cleft regions that facilitates and stabilized cleft formation.63The regenerative potential of the embryonic tissue is striking; even dissociated epithelial cells can self-organize and undergo branching morphogenesis to form tissues with structural features and differentiation markers characteristic of the intact gland.64 Recently, Ogawa et al.65 demonstrated the full functional regeneration of a salivary gland through the orthotopic transplantation of a bioengineered salivary gland germ, reconstituted with epithelial and mesenchymal single cells isolated from the mouse gland germ at embryonic day 13.5–14.5. The bioengineered germ develops into a mature, innervated gland with functional acini, capable secreting salivary fluid that can protect against oral bacterial infections and can effectively restore normal swallowing in a salivary gland-defective mouse model. Although this study provides a proof-of-concept bioengineering approach for the treatment xerostomia, the lack of human embryonic tissues prohibits widespread application of such a strategy for patients with xerostomia.
Available biomaterials that support branching morphogenesis of embryonic salivary glands include PVDF,66 chitosan,67,68 alginate gel,69 fibrous PLGA scaffold,26 and polyacrylamide gel.16 For alginate and polyacrylamide gels, surface conjugation with a cell adhesive peptide (RGD) or fibronectin is necessary to improve cell/tissue adhesion. Not surprisingly, substrate stiffness affects branching morphogenesis.69,70 In general, softer gels (alginate69 or polyacrylamide,16) enhance bud expansion and cleft formation, whereas stiffer gels attenuate them (Fig. 6). Glands cultured on soft gels (4 kPa for alginate gels, and 0.48 kPa for polyacrylamide gels) better resemble developing glands both morphologically and phenotypically, assessed by expression of differentiation markers reflecting various cells in the gland. On stiff gels (184 kPa for alginate gels and 20 kPa for polyacrylamide gels), however, tissue morphology, as well as the expression and distribution of smooth muscle α-actin and AQP5 were altered. Transfer of glands from stiff to soft gels or the addition of exogenous growth factors, such as fibroblast growth factors (FGF 7/10) or transforming growth factor β1 (TGF-β1), resulted in substantial recovery or partial rescue of acinar structure and differentiation. These results indicate that mechanical environments, in addition to chemical signals, should be modeled to better promote organ development in the contexts of salivary gland tissue engineering.
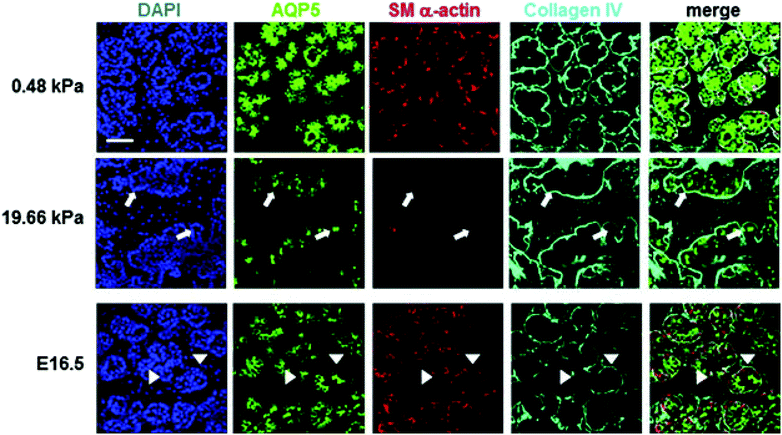 | ||
| Fig. 6 Effect of substrate stiffness on the morphology and cell arrangement of ex vivo cultured embryonic gland. Representative confocal images were captured from the center of organ explants. Collagen IV (cyan) delineates the boundary of the rounded buds, SM α-actin (red) indicates the location of the myoepithelial cells and AQP5 (green) stains for the proacinar/acinar cells. Compared to those cultured on a more compliant (0.48 kPa) substrate and glands developed in vivo (embryonic day 16.5, E16.5), explants cultured on stiff (19.66 kPa) substrates exhibit inconsistent, less organized gland morphology, less homogeneous bud structures, decreased expression of AQP5 and SM α-actin, and aberrant acinar structures lacking SM α-actin-positive cells (white arrows). In the gland grown in vivo, AQP5 is localized apically with the inner epithelial cells (green), highlighted by arrow heads, SM α-actin (red) is expressed in the outer cuboidal cells of the proacinar structures, interior to the basement membrane, as detected by anti-Col IV antibody (cyan). Scale bar = 50 μm. Reprinted from Peters et al., 201416 with permission. | ||
4. Strategies, challenges and future directions
Challenges lie ahead for each approach to the in vitro production of a prototype replacement salivary gland (Fig. 2). In the first envisioned approach that relies on a blind-end tube and a monolayer of duct cells, scaffolds with porous/fibrous features and immobilized basement membrane signals on the luminal surface are conducive to the growth of a cohesive cell monolayer with intimate cell–cell junctions. The inability of ductal cells to secrete proteins and fluid into the lumen of the tubular device, combined with the adverse tissue responses to implanted scaffolds, posed a significant challenge for the clinical translation of such a device. Gene transfer of cDNAs encoding various water channel proteins and secretory proteins is being exploited to install the secretory functions in ductal cells.9,71 To date, it is not clear how to stably and efficiently transfer multiple genes to isolated ductal cells. The safety concerns over the usage of viral vectors and genetically manipulated cells add another level of complexity. Moreover, studies have shown that the implantation of cell-free tubular PLA or PLGA devices elicited moderate inflammation and adverse wound healing responses that may destroy the lining salivary gland cells or plug the open ends. The breakdown of both the polymeric scaffolds adjacent to the oral mucosa, along with the potential for associated damage to the lining graft cells, could provide a source for local mucosal immune challenge.72 Owing to these complications and the lack of validation data, this strategy that relies on the reengineering of ductal cells for the creation of a secretory device has largely been abandoned.The second approach aims at establishing a functional secretory construct using isolated acinar (or stem/progenitor) cells and bioactive scaffolds. Obviously, both mechanical and chemical/biochemical parameters affect cell assembly and organogenesis.69,73,74 Unfortunately, synthetic matrices that foster selective differentiation and organization of multiple cell types have not yet been developed. At a more fundamental level with regard to the hydrogel design, several materials parameters must be considered. When dispersed as single cells during the initial gelation process, salivary gland epithelial cells do not assemble into organized acinar-like structures in stably and densely crosslinked hydrogels as they cannot proliferate and migrate towards each other in such networks.41 Additionally, healthy adult salivary epithelial cells do not actively engage in the remodeling of their surrounding stromal environment.75–77 A more permissive network structure can be generated by tuning the crosslinker length, the linker chemistry, the degree of crosslinking and the network connectivity. Non-covalent interactions78 or reversible covalent bonds79,80 can be introduced to impart dynamic properties to the matrices without compromising the network integrity. A programmed introduction of various biological signals, in a multivalent fashion,81,82 can promote the desired cellular functions at different stages of tissue assembly.83
An alternate strategy for salivary gland tissue engineering is to create well-defined 3D acinar spheroids with close and intimate cell–cell contacts using micro-fabricated templates.84,85 Entrapping these pre-formed spheroids in a synthetic matrix along with other types of cells found in the salivary gland exhibiting matrix remodeling capacity to establish a 3D co-culture system is an attractive strategy to overcome the network restriction and to foster the functionally stable co-assembly of tissue structures.86 As discussed above, fibrous scaffolds mimic the basement membrane morphologically; however, cells directly plated on the scaffold are essentially cultured on 2D. One can apply materials fabrication techniques to introduce macroscopic, interconnected channels or pores within the fibrous scaffolds.87 Cells residing in the macropores or channels are surrounded by the fibrous mesh. As the cells assemble and connect within the scaffold, an integrated 3D construct can be generated and manipulated.88
If appropriately polarized secretory acini are produced, the next technical hurdle is the replication of the ordered and highly branched tissue architecture. So far, branching morphogenesis has been reproduced in vitro using embryonic submandibular gland bud,11,67 but not yet reproduced using human salivary gland epithelial cells isolated from adult tissues. To overcome this technical hurdle, it is tempting, from a materials perspective, to further introduce more complicated molecular and physical information coded in the native tissue to the synthetic scaffolds. However, for clinical translation of tissue engineering products, cost-effectiveness, scalability and the ease of production must be considered.88 In this context, a realistic and immediate goal is to produce the construct containing numerous secretory acini, that once implanted, will reconnect to the ducts that are spared by radiation therapy.1
The third approach harvests the regenerative potential of embryonic tissues. On this front, biomaterials can be designed with the appropriate stiffness and biological signals to maintain the appropriate cell phenotypes, to accelerate the branching morphogenesis and to ensure appropriate spatial organization of multiple cell types in the developing gland. Although the embryonic stem cells/tissues have a significant potential to generate various tissues, their application in tissue engineering is restricted owing to ethical and safety concerns. Recent identification of stem/progenitor cell populations in the adult salivary gland offers opportunities to generate all cell types present in the gland via programmed differentiation.89–91 Still, the establishment of a fully functional gland requires additional methods for isolation, purification and expansion of other types of supporting cells found in the gland.
In all three approaches, the implanted tissue ideally should be vascularized and innervated by the host tissue so that the neotissue receives sufficient oxygen and nutrients, and the secretory function can be controlled by an integrated host nervous system. Overall, tissue engineering of salivary gland is scientifically and technically challenging. More concerted efforts from investigators with diverse backgrounds are needed to make construction of an engineered salivary gland a reality.
5. Conclusions
In this mini review, we describe the structure and the function of salivary gland and outline biomaterials-based strategies for salivary gland tissue engineering. We discuss the limitations of the current materials platforms. Despite these present obstacles, the prospects for tissue engineering with the use of biomimetic scaffolds offer distinct advantages for long term functional restoration of salivary glands. Functional neotissue derived from autologous cells seeded in a network of modified scaffolds could be implanted in a patient with potentially minimal immunogenic risk. Nonetheless, the rate of recent progress is impressive, and there remains a high likelihood that at least one of these strategies will provide useful new avenues to generate glandular tissue replacements for patients with xerostomia.Acknowledgements
Work in the authors’ laboratories has been funded by grants from the National Science Foundation (DMR/BMAT 1206310), National Institutes of Health (R01 DE022969). We acknowledge the Delaware COBRE program (NIGMS: P30 GM110758) for instrumentation support. The authors wish to acknowledge Genzyme for generously providing HA. X. J. acknowledges funding support from DuPont (DuPont Young Professor).References
- R. L. Witt, Salivary Gland Diseases: Surgical and Medical Management, Thieme, New York, 2005 Search PubMed.
- D. A. Harrington, M. Martinez, D. Wu, S. Pradhan-Bhatt and M. C. Farach-Carson, in Advances in Salivary Diagnostics, ed. C. F. Streckfus, Springer, Berlin, Heidelberg, 2015, pp. 157–185 Search PubMed.
- R. M. Nagler and B. J. Baum, Arch. Otolaryngol., Head Neck Surg., 2003, 129, 247–250 CrossRef PubMed.
- D. J. Aframian, E. Cukierman, J. Nikolovski, D. J. Mooney, K. M. Yamada and B. J. Baum, Tissue Eng., 2000, 6, 209–216 CrossRef CAS PubMed.
- S. Pradhan-Bhatt, K. Cannon, D. Zakheim, D. A. Harrington, R. L. Duncan, X. Jia, M. C. Farach-Carson and R. L. Witt, in Stem Cell Biology and Tissue Engineering in Dental Sciences, ed. A. P. S. Vishwakarma, S. Sharpe and M. Ramalingham, Elsevier, 2014, pp. 613–623 Search PubMed.
- I. M. Lombaert, S. M. Knox and M. P. Hoffman, Oral Diseases, 2011, 17, 445–449 CrossRef CAS PubMed.
- W. M. Knosp, S. M. Knox and M. P. Hoffman, Wiley Interdiscip. Rev.: Dev. Biol., 2012, 1, 69–82 CrossRef CAS PubMed.
- V. N. Patel and M. P. Hoffman, Semin. Cell Dev. Biol., 2014, 25, 52–60 CrossRef PubMed.
- D. J. Aframian and A. Palmon, Tissue Eng., Part B, 2008, 14, 187–198 CrossRef PubMed.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, in Molecular Biology of the Cell, Garland Science, New York, 4th edn, 2002, pp. 1065–1125 Search PubMed.
- V. N. Patel, I. T. Rebustini and M. P. Hoffman, Differentiation, 2006, 74, 349–364 CrossRef CAS PubMed.
- N. E. Vrana, P. Lavalle, M. R. Dokmeci, F. Dehghani, A. M. Ghaemmaghami and A. Khademhosseini, Tissue Eng., Part B, 2013, 19, 529–543 CrossRef CAS PubMed.
- R. T. Chitturi, V. Veeravarmal, R. M. Nirmal and B. V. Reddy, J. Clin. Diagn. Res., 2015, 9, ZE14–ZE18 CrossRef PubMed.
- J. Harunaga, J. C. Hsu and K. M. Yamada, J. Dent. Res., 2011, 90, 1070–1077 CrossRef CAS PubMed.
- S. Pradhan-Bhatt, D. A. Harrington, R. L. Duncan, X. Jia, R. L. Witt and M. C. Farach-Carson, Tissue Eng., Part A, 2013, 19, 1610–1620 CrossRef CAS PubMed.
- S. B. Peters, N. Naim, D. A. Nelson, A. P. Mosier, N. C. Cady and M. Larsen, Tissue Eng., Part A, 2014, 20, 1632–1642 CrossRef CAS PubMed.
- Y. H. Chan, T. W. Huang, Y. S. Chou, S. H. Hsu, W. F. Su, P. J. Lou and T. H. Young, Biomaterials, 2012, 33, 464–472 CrossRef CAS PubMed.
- D. J. Aframian, S. D. Tran, E. Cukierman, K. M. Yamada and B. J. Baum, Tissue Eng., 2002, 8, 871–878 CrossRef CAS PubMed.
- M. H. Chen, Y. H. Hsu, C. P. Lin, Y. J. Chen and T. H. Young, J. Biomed. Mater. Res., Part A, 2005, 74, 254–262 CrossRef PubMed.
- B. X. Zhang, Z. L. Zhang, A. L. Lin, H. Wang, M. Pilia, J. L. Ong, D. D. Dean, X. D. Chen and C. K. Yeh, Tissue Eng., Part A, 2015, 21, 1611–1620 CrossRef CAS PubMed.
- M. C. Farach-Carson, A. J. Brown, M. Lynam, J. B. Safran and D. D. Carson, Matrix Biol., 2008, 27, 150–160 CrossRef CAS PubMed.
- S. Pradhan, C. Zhang, X. Jia, D. D. Carson, R. Witt and M. C. Farach-Carson, Tissue Eng., Part A, 2009, 15, 3309–3320 CrossRef CAS PubMed.
- S. Pradhan, C. Liu, C. Zhang, X. Jia, M. C. Farach-Carson and R. L. Witt, Otolaryngol. – Head Neck Surg., 2010, 142, 191–195 CrossRef PubMed.
- O. M. Maria, A. Zeitouni, O. Gologan and S. D. Tran, Tissue Eng., Part A, 2011, 17, 1229–1238 CrossRef CAS PubMed.
- D. Liang, B. S. Hsiao and B. Chu, Adv. Drug Delivery Rev., 2007, 59, 1392–1412 CrossRef CAS PubMed.
- S. J. Sequeira, D. A. Soscia, B. Oztan, A. P. Mosier, R. Jean-Gilles, A. Gadre, N. C. Cady, B. Yener, J. Castracane and M. Larsen, Biomaterials, 2012, 33, 3175–3186 CrossRef CAS PubMed.
- S. I. Cantara, D. A. Soscia, S. J. Sequeira, R. P. Jean-Gilles, J. Castracane and M. Larsen, Biomaterials, 2012, 33, 8372–8382 CrossRef CAS PubMed.
- D. A. Soscia, S. J. Sequeira, R. A. Schramm, K. Jayarathanam, S. I. Cantara, M. Larsen and J. Castracane, Biomaterials, 2013, 34, 6773–6784 CrossRef CAS PubMed.
- M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518–13519 CrossRef CAS PubMed.
- M. T. Taylor, M. L. Blackman, O. Dmitrenko and J. M. Fox, J. Am. Chem. Soc., 2011, 133, 9646–9649 CrossRef CAS PubMed.
- S. Liu, H. Zhang, R. A. Remy, F. Deng, M. E. Mackay, J. M. Fox and X. Jia, Adv. Mater., 2015, 27, 2783–2790 CrossRef CAS PubMed.
- V. Szlavik, J. Vag, K. Marko, K. Demeter, E. Madarasz, I. Olah, T. Zelles, B. C. O'Connell and G. Varga, J. Cell. Biochem., 2008, 103, 284–295 CrossRef CAS PubMed.
- M. Furue, T. Okamoto, H. Hayashi, J. D. Sato, M. Asashima and S. Saito, In Vitro Cell. Dev. Biol.: Anim., 1999, 35, 131–135 CrossRef CAS.
- A. D. McCall, J. W. Nelson, N. J. Leigh, M. E. Duffey, P. Lei, S. T. Andreadis and O. J. Baker, Tissue Eng., Part A, 2013, 19, 2215–2225 CrossRef CAS PubMed.
- A. Joraku, C. A. Sullivan, J. Yoo and A. Atala, Differentiation, 2007, 75, 318–324 CrossRef CAS PubMed.
- Z. Gao, T. Wu, J. Xu, G. Liu, Y. Xie, C. Zhang, J. Wang and S. Wang, Cells Tissues Organs, 2014, 200, 171–180 CrossRef CAS PubMed.
- S. Pradhan-Bhatt, D. A. Harrington, R. L. Duncan, M. C. Farach-Carson, X. Jia and R. L. Witt, Laryngoscope, 2014, 124, 456–461 CrossRef CAS PubMed.
- K. T. Dicker, L. A. Gurski, S. Pradhan-Bhatt, R. L. Witt, M. C. Farach-Carson and X. Jia, Acta Biomater., 2014, 10, 1558–1570 CrossRef CAS PubMed.
- C. Mangold, F. Wurm and H. Frey, Polym. Chem., 2012, 3, 1714–1721 RSC.
- J. Zhu, Biomaterials, 2010, 31, 4639–4656 CrossRef CAS PubMed.
- A. D. Shubin, T. J. Felong, D. Graunke, C. E. Ovitt and D. S. Benoit, Tissue Eng., Part A, 2015, 21, 1733–1751 CrossRef CAS PubMed.
- H. G. Garg and C. A. Hales, Chemistry and Biology of Hyaluronan, Elsevier Ltd, Oxford, 2004 Search PubMed.
- X. Xu, L. A. Gurski, C. Zhang, D. A. Harrington, M. C. Farach-Carson and X. Jia, Biomaterials, 2012, 33, 9049–9060 CrossRef CAS PubMed.
- L. A. Gurski, A. K. Jha, C. Zhang, X. Jia and M. C. Farach-Carson, Biomaterials, 2009, 30, 6076–6085 CrossRef CAS PubMed.
- A. J. Farran, S. S. Teller, A. K. Jha, T. Jiao, R. A. Hule, R. J. Clifton, D. P. Pochan, R. L. Duncan and X. Jia, Tissue Eng., Part A, 2010, 16, 1247–1261 CrossRef CAS PubMed.
- X. Xu, A. K. Jha, D. A. Harrington, M. C. Farach-Carson and X. Jia, Soft Matter, 2012, 8, 3280–3294 RSC.
- S. Liu, K. T. Dicker and X. Jia, Chem. Commun., 2015, 51, 5218–5237 RSC.
- S. E. Grieshaber, A. J. Farran, S. Lin-Gibson, K. L. Kiick and X. Jia, Macromolecules, 2009, 42, 2532–2541 CrossRef CAS PubMed.
- S. E. Grieshaber, A. J. Farran, S. Bai, K. L. Kiick and X. Jia, Biomacromolecules, 2012, 13, 1774–1786 CrossRef CAS PubMed.
- L. Xiao, J. Zhu, D. J. Londono, D. J. Pochan and X. Jia, Soft Matter, 2012, 8, 10233–10237 RSC.
- H. Zhang, K. T. Dicker, X. Xu, X. Jia and J. M. Fox, ACS Macro Lett., 2014, 3, 727–731 CrossRef CAS PubMed.
- X. Jia, Y. Yeo, R. J. Clifton, T. Jiao, D. S. Kohane, J. B. Kobler, S. M. Zeitels and R. Langer, Biomacromolecules, 2006, 7, 3336–3344 CrossRef CAS PubMed.
- N. Sahiner, A. K. Jha, D. Nguyen and X. Jia, J. Biomater. Sci., Polym. Ed., 2008, 19, 223–243 CrossRef CAS PubMed.
- A. K. Jha, W. Yang, C. B. Kirn-Safran, M. C. Farach-Carson and X. Jia, Biomaterials, 2009, 30, 6964–6975 CrossRef CAS PubMed.
- X. Xu, A. K. Jha, R. L. Duncan and X. Jia, Acta Biomater., 2011, 7, 3050–3059 CrossRef CAS PubMed.
- A. K. Jha, R. A. Hule, T. Jiao, S. S. Teller, R. J. Clifton, R. L. Duncan, D. J. Pochan, X. Jia, C. Ni, X. Li, Z. Chen, H. H. Li, I. Shah and J. Q. Xiao, Macromolecules, 2009, 42, 537–546 CrossRef CAS PubMed.
- A. K. Jha, M. S. Malik, M. C. Farach-Carson, R. L. Duncan and X. Jia, Soft Matter, 2010, 6, 5045–5055 RSC.
- A. K. Jha, X. Xu, R. L. Duncan and X. Jia, Biomaterials, 2011, 32, 2466–2478 CrossRef CAS PubMed.
- S. K. Nigam, Stem Cells Transl. Med., 2013, 2, 993–1000 CrossRef CAS PubMed.
- J. S. Harunaga, A. D. Doyle and K. M. Yamada, Dev. Biol., 2014, 394, 197–205 CrossRef CAS PubMed.
- J. C. Hsu, H. Koo, J. S. Harunaga, K. Matsumoto, A. D. Doyle and K. M. Yamada, Dev. Dyn., 2013, 242, 1066–1077 CrossRef CAS PubMed.
- W. P. Daley, K. M. Gulfo, S. J. Sequeira and M. Larsen, Dev. Biol., 2009, 336, 169–182 CrossRef CAS PubMed.
- T. Sakai, M. Larsen and K. M. Yamada, Nature, 2003, 423, 876–881 CrossRef CAS PubMed.
- C. Wei, M. Larsen, M. P. Hoffman and K. M. Yamada, Tissue Eng., 2007, 13, 721–735 CrossRef CAS PubMed.
- M. Ogawa, M. Oshima, A. Imamura, Y. Sekine, K. Ishida, K. Yamashita, K. Nakajima, M. Hirayama, T. Tachikawa and T. Tsuji, Nat. Commun., 2013, 4, 2498 Search PubMed.
- T. L. Yang, Y. C. Hsiao, S. J. Lin, H. W. Lee, P. J. Lou, J. Y. Ko and T. H. Young, Biomaterials, 2010, 31, 288–295 CrossRef CAS PubMed.
- T. L. Yang and T. H. Young, Biomaterials, 2008, 29, 2501–2508 CrossRef CAS PubMed.
- T. L. Yang and Y. C. Hsiao, Biomaterials, 2015, 66, 29–40 CrossRef CAS PubMed.
- H. Miyajima, T. Matsumoto, T. Sakai, S. Yamaguchi, S. H. An, M. Abe, S. Wakisaka, K. Y. Lee, H. Egusa and S. Imazato, Biomaterials, 2011, 32, 6754–6763 CrossRef CAS PubMed.
- S. B. Peters, D. A. Nelson, H. R. Kwon, M. Koslow, K. A. DeSantis and M. Larsen, Matrix Biol., 2015, 43, 109–124 CrossRef CAS PubMed.
- B. J. Baum and S. D. Tran, Periodontology 2000, 2006, 41, 218–223 CrossRef PubMed.
- D. J. Aframian, R. S. Redman, S. Yamano, J. Nikolovski, E. Cukierman, K. M. Yamada, M. F. Kriete, W. D. Swaim, D. J. Mooney and B. J. Baum, Tissue Eng., 2002, 8, 649–659 CrossRef CAS PubMed.
- A. Raza, C. S. Ki and C. C. Lin, Biomaterials, 2013, 34, 5117–5127 CrossRef CAS PubMed.
- C. Barnes, L. Speroni, K. P. Quinn, M. Montevil, K. Saetzler, G. Bode-Animashaun, G. McKerr, I. Georgakoudi, C. S. Downes, C. Sonnenschein, C. V. Howard and A. M. Soto, PLoS One, 2014, 9, e93325 Search PubMed.
- W. P. Daley, S. B. Peters and M. Larsen, J. Cell Sci., 2008, 121, 255–264 CrossRef CAS PubMed.
- E. S. Radisky and D. C. Radisky, J. Mammary Gland Biol. Neoplasia, 2010, 15, 201–212 CrossRef PubMed.
- C. A. DeForest and K. S. Anseth, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 421–444 CrossRef CAS PubMed.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- L. Y. Jiang, B. Lv and Y. Luo, Biomaterials, 2013, 34, 2665–2673 CrossRef CAS PubMed.
- B. W. Han, H. Layman, N. A. Rode, A. Conway, D. V. Schaffer, N. J. Boudreau, W. M. Jackson and K. E. Healy, Tissue Eng., Part A, 2015, 21, 2366–2378 CrossRef CAS PubMed.
- M. W. Tibbitt and K. S. Anseth, Sci. Transl. Med., 2012, 4, 160ps124 Search PubMed.
- F. Pampaloni, E. G. Reynaud and E. H. Stelzer, Nat. Rev. Mol. Cell Biol., 2007, 8, 839–845 CrossRef CAS PubMed.
- S. Sart, A. C. Tsai, Y. Li and T. Ma, Tissue Eng., Part B, 2014, 20, 365–380 CrossRef PubMed.
- E. Fennema, N. Rivron, J. Rouwkema, C. van Blitterswijk and J. de Boer, Trends Biotechnol., 2013, 31, 108–115 CrossRef CAS PubMed.
- G. Wei and P. X. Ma, Biomaterials, 2009, 30, 6426–6434 CrossRef CAS PubMed.
- E. S. Place, N. D. Evans and M. M. Stevens, Nat. Mater., 2009, 8, 457–470 CrossRef CAS PubMed.
- S. Pringle, R. Van Os and R. P. Coppes, Stem Cells, 2013, 31, 613–619 CrossRef CAS PubMed.
- C. Yoo, J. B. Vines, G. Alexander, K. Murdock, P. Hwang and H. W. Jun, Biomater. Res., 2014, 18, 9 CrossRef PubMed.
- S. I. Jang, H. L. Ong, A. Gallo, X. Liu, G. Illei and I. Alevizos, J. Dent. Res., 2015, 94, 304–311 CrossRef CAS PubMed.
- S. Wang, E. Cukierman, W. D. Swaim, K. M. Yamada and B. J. Baum, Biomaterials, 1999, 20, 1043–1049 CrossRef CAS PubMed.
- H. Gray, P. L. Williams and L. H. Bannister, Gray's Anatomy: the Anatomical Basis of Medicine and Surgery, Churchill Livingstone, New York, 1995 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |