Electric field-mediated growth of osteoblasts – the significant impact of dynamic flow of medium
A.
Kumar
,
K. C.
Nune
and
R. D. K.
Misra
*
Department of Metallurgical, Materials and Biomedical Engineering, University of Texas at El Paso, El Paso, TX 79968, USA. E-mail: dmisra2@utep.edu
First published on 14th October 2015
Abstract
The endogenous electric field plays an important role in accomplishing various functions including communication with the brain and with different parts of the physiological system, wound healing, and cellular functions. Furthermore, the endogenous electric field can be modified using the external electric field to induce changes in cell functionality. Given that the cells grow in contact with the dynamic flow of blood and nutrients, the objective of the study is to elucidate the effect of media flow (dynamic conditions) on osteoblast functions at a pulsed DC (direct current) electric field of strength of 0.5–1 V cm−1 and compared with the static conditions (no flow of media and in the presence of an electric field). The electric field provided a guiding cue to cells to move towards the cathode. An interesting aspect of the electric field was the migration of cells towards the cathode with the axis parallel to the direction of the electric field such that the lamellipodia was aligned. Furthermore, there was an absence of membrane blebbing or necrosis at the cathode. However, cell growth and expression of proteins (actin and vinculin) were higher than the anode. In contrast, at the anode, while the cells were healthy, the cell growth was less such that the expression of vinculin was relatively low together with less densely packed actin stress fibers. It is underscored that the biological functionality is favorably altered in the presence of an electrical field under dynamic conditions with a consequent effect on cell proliferation, growth, and expression level of prominent proteins, actin and vinculin.
1. Introduction
The biological systems such as cells and tissues are responsive to the endogenous cues and external stimuli.1–3 From this perspective, the external electric field appropriate to the physiological environment can be used to regulate the cellular and/or tissue functions.4 For example, the external electric field was observed to be effective in the reorganization of actin filaments,5,6 cell motility,7 guidance of neuronal growth cone,8 and stem cell differentiation into various lineages.9,10 Thus, external stimuli can be effectively used to override the endogenous cues and cure the relevant disease. The electric field was beneficial in tissue and nerve regeneration as well as in cancer therapy.11–15 The effect of the electric field on different cells has different effects because of the variation in the physiological characteristics of cells. For example, in differentiated osteoblasts, the attachment of the plasma membrane with the cytoskeleton is stronger than in undifferentiated stem cells. Thus, they respond differently to the external electric field.16 In one study, Jurkat cells experienced cell membrane damage when a short electric field pulse (1–10 sequential pulses) was applied, while human blood polymorphonuclear leucocytes were unaffected under similar conditions.17 It is important to indicate that the sub-microsecond intense pulsed electric field can lead to permeabilization of intracellular membrane without causing electroporation and cells may die through apoptosis.18,19More importantly, a short duration pulse eludes the probability of overheating of cells as the electric field energy required for the cell lysis is reduced with a decrease in pulse duration.20 An increase in cell proliferation and spreading on the biomaterial surface was observed in a narrow window of DC (direct current) pulsed electric field (667 mV cm−1 and 100 Hz in the case of glass).21 In the case of hydroxyapatite (HA) and HA-40 wt% barium titanate, at 100 Hz frequency, the optimal electric fields were 167 and 667 mV cm−1.21 No evidence of heating of the medium was noted. The electric field led to an increase in osteoblast proliferation by 72% when applied for 1 h for 5 days.22 Interestingly, under the effect of the electric field, the osteoblasts migrated toward the cathode, while osteoclasts migrated toward the anode.23
Given that the cells are dielectric in nature, the frequency and electric field strength influence the strength of the electric field experienced by the cells. It is important to mention that the optimal stimulation parameters are a function of biomaterial surface and properties of the culture medium.21,24 With regard to the effect of properties of the culture medium on the electric field stimulated cell behavior, the electric field led to build-up of electrical charge at the cell membrane in a conducting medium. This induced a change in the voltage across the plasma membrane and subsequent opening of plasma membrane channels (in the presence of a low strength electric field), referred to as voltage gating.20 This causes cells to be in the state of stress because of change in the ion concentration inside the cells induced by flux of ions such as Na+ and K+.
Based on the above discussion, it is clear that the electric field can be used to modulate cell function. Thus, it is important to understand the cell behavior under the influence of the electric field. In a physiological environment, the flow of blood and nutrients along with the endogenous electric field are involved in tissue function. With the above in mind, it is desirable to study the effect of the electric field on cell functionality under the dynamic conditions, i.e. during the flow of media. The previous studies were focused on cell behavior when the culture medium was static.21,24,25 In the present study, our objective is to study the effect of the electric field on osteoblast functions at electric field strengths of 0.5 and 1 V cm−1 under dynamic conditions and compare that with the static conditions.
2. Materials and methods
2.1 Starting material and electric field stimulation
The glass cover slips (Cat no. 63768-01, Gold-Seal, USA) of dimensions (24 × 40 mm) and ∼2 mm thickness were used as a substrate for the cells. Glass cover slips were ultrasonicated in acetone and then in 70% ethanol for 10 min each. The samples were marked for the direction of the electric field and flow of media using an alcohol proof marker. These samples were autoclaved and kept for further study. The osteoblasts (MC3T3-E1, Cat. no. CRL-2593, ATCC, USA) with a cell density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well (for sparse cells) as well as 30
000 cells per well (for sparse cells) as well as 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well (for monolayers) were seeded on the sample surface in a non-treated 8-well plate (Cat. no. 267062, Nunc, USA, well dimension: 3 × 3.9 mm). After 1 day of incubation, cells seeded on cover slips were stimulated for 10 min by pulsed DC electric fields of 0.5 V cm−1 and 1 V cm−1 strength (square waveform, 100 Hz frequency and 50% duty cycle). The experimental plan is presented in Table 1. A function generator (model no. AFG3022C, Tektronix, USA) was used to apply the electric field. Stainless steel (ASTM 316L) electrodes were used in this study to avoid the degradation of the electrode due to corrosion. Electrodes were cleaned in acetone and autoclaved after each experiment. Media (1 × PBS (phosphate buffered saline)) flow (10 ml min−1) was maintained during the entire period of electric field application to mimic the dynamic conditions of the physiological system. For this, a custom designed bioreactor equipped with a peristaltic pump (model no. 07522-20, Cole Parmer, USA) was used (Fig. 1). For comparison, an electric field was also applied on the samples under the static conditions, i.e. no flow of medium. The entire process of electric field stimulation under dynamic as well as static conditions was repeated every day for a total period of 5 days. After 5 days, the samples were incubated for 1 day prior to the assessment of cell viability.
000 cells per well (for monolayers) were seeded on the sample surface in a non-treated 8-well plate (Cat. no. 267062, Nunc, USA, well dimension: 3 × 3.9 mm). After 1 day of incubation, cells seeded on cover slips were stimulated for 10 min by pulsed DC electric fields of 0.5 V cm−1 and 1 V cm−1 strength (square waveform, 100 Hz frequency and 50% duty cycle). The experimental plan is presented in Table 1. A function generator (model no. AFG3022C, Tektronix, USA) was used to apply the electric field. Stainless steel (ASTM 316L) electrodes were used in this study to avoid the degradation of the electrode due to corrosion. Electrodes were cleaned in acetone and autoclaved after each experiment. Media (1 × PBS (phosphate buffered saline)) flow (10 ml min−1) was maintained during the entire period of electric field application to mimic the dynamic conditions of the physiological system. For this, a custom designed bioreactor equipped with a peristaltic pump (model no. 07522-20, Cole Parmer, USA) was used (Fig. 1). For comparison, an electric field was also applied on the samples under the static conditions, i.e. no flow of medium. The entire process of electric field stimulation under dynamic as well as static conditions was repeated every day for a total period of 5 days. After 5 days, the samples were incubated for 1 day prior to the assessment of cell viability.
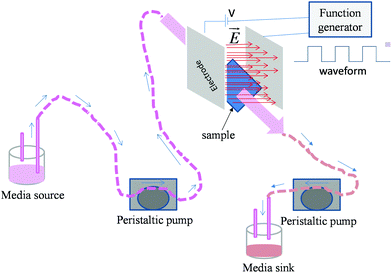 | ||
| Fig. 1 Schematic representation of experimental set-up used for the electric field stimulation of osteoblasts in dynamic condition. | ||
| Culture conditions | Applied electric field (V cm−1) | |||||
|---|---|---|---|---|---|---|
| Static | Dynamic | |||||
| No electric field | Electric field | No electric field | Electric field | |||
| Monolayer | 0 | 0.5 | 1 | 0 | 0.5 | 1 |
| Sparse | 0 | 1 | 1 | |||
2.2. Cell viability and cytocompatibility
To study cell viability in the presence of electric field and flow of media, osteoblasts were seeded on the glass cover slip for 1 day. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 30
000 and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml were seeded on individual scaffolds and pre-incubated for 4 h at 37 °C in an environment of 5% CO2 and 95% relative humidity. After the pre-incubation period, complete culture media (89% α MEM (minimum essential medium), 10% FBS (fetal bovine serum), and 1% antibiotic) were added and the samples were incubated for 1 day before the electric field stimulation. The electric field was applied for 10 minutes under both static and dynamic conditions each day and for 5 days. At the end of second day, the old media was replaced by fresh media, to avoid cell death because of change in the pH of the media induced by cellular activity. After 5 days of application of the electric field, the samples were incubated for another 1 day to normalize the cells. Next, the samples were washed with 1 × PBS and fixed with 4% formaldehyde, followed by cell wall permeabilization with 0.1% triton × 100. To reduce the non-specific binding of dyes, the samples were incubated with 5% FBS. After washing with 1 × PBS, cells were stained with dyes to observe the expression of vinculin (green color) and actin (red color). DAPI was used to stain the nucleus (blue color). After staining, the samples were washed with 1 × PBS to remove the unreacted dye and stored in 1 × PBS at 6 °C for florescence microscopy. Expression of vinculin and actin was used to study the cell adhesion (focal adhesion) and cytoskeleton reorganization, respectively. Cell viability was studied through visualization of nuclei.
000 cells per ml were seeded on individual scaffolds and pre-incubated for 4 h at 37 °C in an environment of 5% CO2 and 95% relative humidity. After the pre-incubation period, complete culture media (89% α MEM (minimum essential medium), 10% FBS (fetal bovine serum), and 1% antibiotic) were added and the samples were incubated for 1 day before the electric field stimulation. The electric field was applied for 10 minutes under both static and dynamic conditions each day and for 5 days. At the end of second day, the old media was replaced by fresh media, to avoid cell death because of change in the pH of the media induced by cellular activity. After 5 days of application of the electric field, the samples were incubated for another 1 day to normalize the cells. Next, the samples were washed with 1 × PBS and fixed with 4% formaldehyde, followed by cell wall permeabilization with 0.1% triton × 100. To reduce the non-specific binding of dyes, the samples were incubated with 5% FBS. After washing with 1 × PBS, cells were stained with dyes to observe the expression of vinculin (green color) and actin (red color). DAPI was used to stain the nucleus (blue color). After staining, the samples were washed with 1 × PBS to remove the unreacted dye and stored in 1 × PBS at 6 °C for florescence microscopy. Expression of vinculin and actin was used to study the cell adhesion (focal adhesion) and cytoskeleton reorganization, respectively. Cell viability was studied through visualization of nuclei.
3. Results
3.1. Effect of electric field on osteoblast monolayer under static and dynamic conditions
Experiments were carried out under two culture conditions, namely monolayer and sparse. In the case of monolayer, a high density of cells (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml) was seeded, while for sparse culture, the seeding density was 10
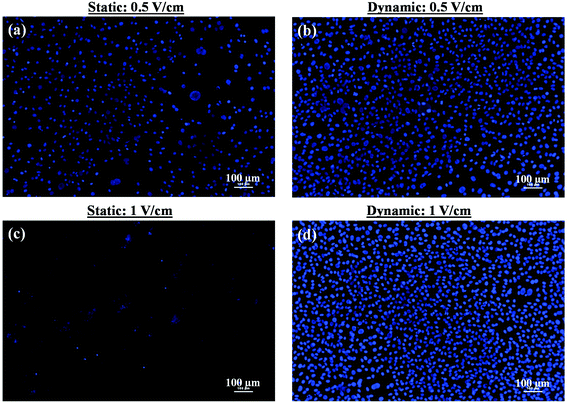
000 cells per ml) was seeded, while for sparse culture, the seeding density was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml. In the former case, the high cell density enabled the cell-to-cell contact after 1 day of culture. In contrast, in the latter case, because of low cell seeding density, the cell-to-cell contact was not observed after the first day of incubation. We first summarize the results related to higher seeding density. In this condition, both under the static and dynamic conditions, normal cell growth (increase in cell density with time) was observed in the absence of an electric field. The cells were healthy even after 5 days of culture. However, after five days of static culture and exposure to 0.5 V cm−1 electric field for 10 min each day, few unhealthy cells were noted, characterized by fragmented nucleus. Also, a low cell number (or nucleus count) (Fig. 2a and Table 2) was observed, presumably because of low cell viability. In contrast, no significant damage to cells was induced by 0.5 V cm−1 electric field under the dynamic conditions (Fig. 2b and Table 2).
000 cells per ml. In the former case, the high cell density enabled the cell-to-cell contact after 1 day of culture. In contrast, in the latter case, because of low cell seeding density, the cell-to-cell contact was not observed after the first day of incubation. We first summarize the results related to higher seeding density. In this condition, both under the static and dynamic conditions, normal cell growth (increase in cell density with time) was observed in the absence of an electric field. The cells were healthy even after 5 days of culture. However, after five days of static culture and exposure to 0.5 V cm−1 electric field for 10 min each day, few unhealthy cells were noted, characterized by fragmented nucleus. Also, a low cell number (or nucleus count) (Fig. 2a and Table 2) was observed, presumably because of low cell viability. In contrast, no significant damage to cells was induced by 0.5 V cm−1 electric field under the dynamic conditions (Fig. 2b and Table 2).
| 0 V cm−1 | 0.5 V cm−1 | 1 V cm−1 | ||||
|---|---|---|---|---|---|---|
| Static | Dynamic | Static | Dynamic | Static | Dynamic | |
| Day 1 | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells |
| Day 2 | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells |
| Day 3 | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells |
| Day 4 | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells | Healthy cells |
| Day 5 | Healthy cells | Healthy cells | Cells were not healthy | Healthy cells | Cells were dying | Healthy cells |
When the electric field was increased to 1 V cm−1, a strong detrimental effect of the electric field was observed under the static conditions. Majority of the cells were dead after the five days of electric field stimulation. This was confirmed by the presence of cell debris and fragmented nuclei (Fig. 2c). In contrast, the cells were found healthy after five days of culture and nucleus count was higher under the dynamic conditions (Fig. 2d).
3.2. Effect of electric field on sparse osteoblasts under static and dynamic conditions
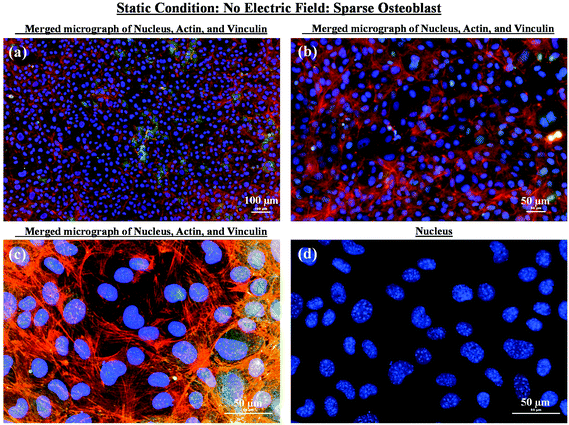
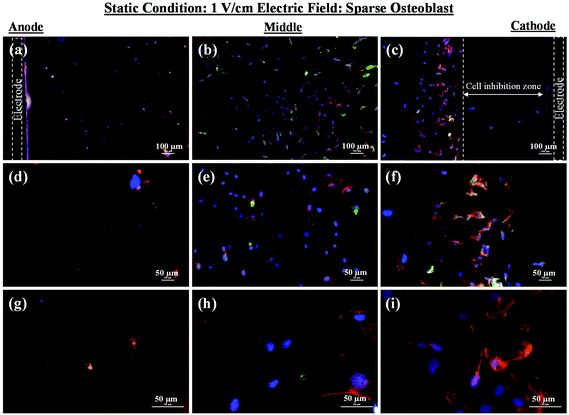
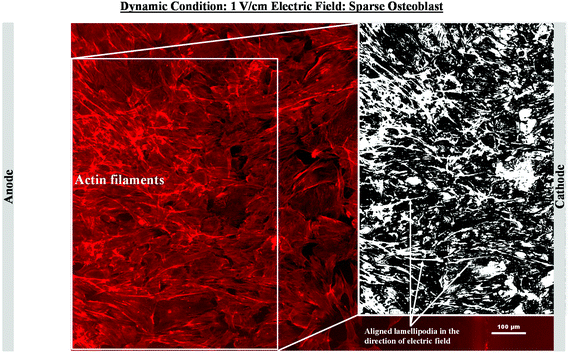
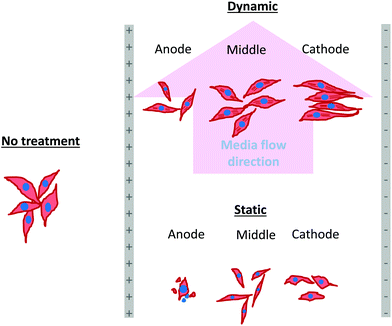
As mentioned in the previous section, in the case of osteoblast monolayer, the maximum damage was observed at 1 V cm−1 electric field, when the static conditions were maintained during electric field application. In order to further extend our understanding with respect to cell seeding density and flow of media on electric field-mediated cell growth, we seeded low density of cells to avoid the formation of a confluence cell layer on the material surface. Here the absence of an electric field and static conditions was considered as the control. High cell viability was observed on control samples after 5 days of culture (Fig. 3a and b). Extensive reorganization of actin filaments (red color) and higher expression of vinculin (green color) were noted at higher magnification (Fig. 3c). Also, no fragmentation of cell nucleus was observed (Fig. 3d).Intriguing observations were made under static and dynamic conditions in the presence of an electric field (Fig. 4 and 5). Under static conditions, a low density of cells was observed in the vicinity of the anode and cathode (Fig. 4). The low density of cells is attributed to cell damage/death induced by the electric field. The damage was more uniform in the proximity of the anode. In contrast, in the vicinity of the cathode, a relatively larger number of cells were observed, except in the region close to the electrode (we refer to this zone as the cell-inhibition zone). In this zone no signature of cells was noted. Interestingly, no cell-inhibition zone was observed in the anode region. Importantly, very distinct cell morphology was found in the three regions of the substrate: (a) vicinity of anode, (b) vicinity of cathode and (c) mid-region of the sample. In the mid-region, cell growth was observed as interpreted from extended lamellipodia. The higher magnification micrographs of regions close to the cathode indicated an alignment of lamellipodia in the direction of the electric field, which is a distinct evidence of cell migration. However, no distinct evidence of directional migration of cells was noticed in the mid-region. Also, cell spreading was more in the mid-region as compared to the regions in the vicinity of the electrodes.
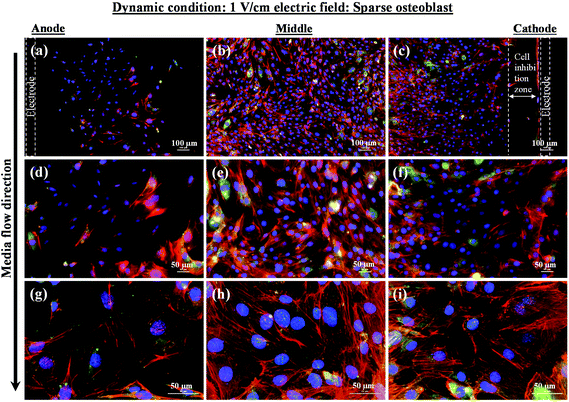
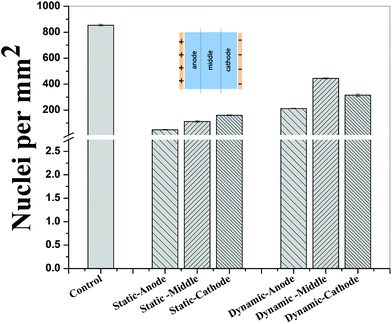
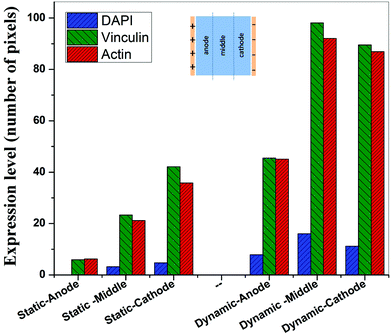
In contrast to the static conditions, the dynamic conditions was observed to be relatively more viable for electric field-mediated cell growth based on the higher density of viable cells under the dynamic conditions (Fig. 5). However, cell morphology was similar in all the three regions: proximity of the anode and cathode, and the mid region of the sample. Similar to static conditions, lower number of cells were observed in the vicinity of the anode. However, under the dynamic conditions, cells were relatively healthier than the static conditions. The higher magnification micrographs confirmed the organization of actin and noticeable expression of vinculin. In the vicinity of the cathode, the cell-inhibition zone was very small and a high density of cells was observed near to this zone because of enhanced migration of cells towards the cathode and alignment of cells in the direction of the electric field. Low and high magnification micrographs clearly revealed the extension of lamellipodia in the direction of the electric field, towards the cathode (Fig. 5 and 6). Importantly, in both middle and cathode regions, extensive growth of cells with the reorganization of actin and high expression of vinculin were observed. A schematic of the response of cells under the static and dynamic conditions is presented in Fig. 7. Fig. 8 and 9 summarize the quantitative data in terms of number of cells and the expression level of proteins for static and dynamic culture conditions. Fig. 8 shows the number of cells per unit area under static and dynamic conditions. The results were compared with the control sample (in the absence of an electric field under static conditions). Results revealed reduced proliferation of osteoblasts in the presence of an electric field strength of 1 V cm−1, irrespective of static or dynamic conditions. However, as compared to static conditions, higher number of cells were found under dynamic conditions. The quantitative analysis of cumulative expression of vinculin and actin indicated the benefits of dynamic conditions over static conditions (Fig. 9). In general, the higher value of DAPI under dynamic conditions confirmed higher cell growth as compared to the static conditions. Also, the higher expression level of vinculin and actin confirmed stronger cell adhesion and proliferation of cells under the dynamic conditions as discussed in detail in section 4.
4. Discussion
The external electric field has beneficial effects in the context of physiological activity such as enhanced synthesis of proteins. The activity at the molecular scale plays an important role in the cell fate process: cell cycle, proliferation, and apoptosis. The wound healing process can be accelerated by electric field-mediated cell migration towards the wound region. The cell migration is a result of force applied by the electric field on the naturally charged cell surface and the direction of the electric field is the direction of force applied on a positive charge. In reality, a live cell is a conducting mass consisting of a conducting cytoplasm (∼10−2 S cm−1) and a cell membrane (phospholipid bilayer) of lower conductivity (∼10−3 S cm−1).26,27 Thus, cells get polarized in the presence of an electric field and act as a dipole. The application of the electric field in orthopedic applications promotes bone regeneration and can be used to treat osteoporosis and fracture of bones.28–30 In one instance of the guinea pig spinal cord, the dorsal column fibres were recovered by an electric field (∼300–400 mV mm−1).31,32Previous studies on the effect of the electric field on cell behavior in vitro were performed under static conditions.21,24,25 However, in these experiments, the effect of properties of culture media on cell metabolism was not systematically studied. For instance, during the application of electric field, the variation in pH and denaturing of proteins and the effect of these parameters on the cell behavior were not considered. We have considered these aspects by maintaining the media flow during electric field application, which ensures continuous supply of fresh media to the cells. Moreover, in natural tissue there is continuous flow of blood that ensures homeostatic conditions, which was ignored in the previous studies during the application of electric field under the static conditions (no flow of media).21,24,25 Thus, we have maintained a continuous flow of the media to minimize change in pH and denaturing of proteins (Fig. 1). In the present study, 1 × PBS was used as a medium during electric field application.
In an earlier study, the application of 2 V cm−1 electric field for 60 min in a serum free buffer saline led to partial disassembly of actin in osteoblasts as well as hMSCs4 and there was a two-fold decrease in cell elasticity. This also led to depletion in intracellular ATP (adenosine triphosphate). The depletion of ATP resulted in physical decoupling of the cell membrane with the cytoskeleton. Furthermore, the depletion of ATP led to the inhibition of linker proteins that couple the cell membrane with the cytoskeleton. Disruption of the actin filament is a clear indication of dissociation of the cell membrane from the cytoskeleton.33 The separation of the cell membrane from the cytoskeleton induced by the electric field led to cell membrane blebbing.34 Similar results were observed in our study when 1 V cm−1 electric field was applied on sparse cells under the static conditions. Interestingly, the effect of membrane blebbing was more prominent near the electrodes (Fig. 4). Moreover, a severe damage to the cells was found near the anode, which may be considered due to necrosis of cells. In the middle region, although disruption of actin filaments was not noticed, a less densely packed thin bundle of actin fibers was present, which is a clear indication of poor cell adhesion and growth. In contrast, under the dynamic conditions, no signature of membrane blebbing or cell necrosis was observed (Fig. 5). However, a relatively low cell density was observed in the vicinity of the anode. Also, the cells in the vicinity of the anode were characterized by healthy nucleus with moderately high expression level of vinculin and less densely packed bundle of acting filaments. In contrast, in the vicinity of the cathode and mid-region, the cell growth was higher than the anode. Also, the densely packed thick bundle of actin filaments and enhanced expression of vinculin were observed. Importantly, the actin filaments were found arranged in the direction of the electric field in the proximity of the cathode (Fig. 6). This confirmed the migration of cells towards the cathode or alignment of cells in the direction of the electric field near the cathode region. It is important to mention that the electric field affects the direction of cell motility than the speed and provides a guiding cue to cells to move towards the cathode.7 As a consequence, the cathode facing the cells move into the wound more rapidly under the effect of the electric field, while the anode facing cells did not show any movement.7
Cellular processes such as proliferation depend on DNA synthesis. DNA synthesis was found to be accelerated in the presence of an electric field.35 In the context of a molecular mechanism involved in cell proliferation, the electric field activates extracellular signaling molecules, which is further transferred to the intracellular signaling proteins via molecular switch.36 The electric field induced signaling was observed to be effective in the proliferation of fibroblasts on glass, hydroxyapatite, and hydroxyapatite–barium titanate samples in the presence of a pulsed DC electric field in the range of 0.3–0.6 V cm−1.21 The number of cells per unit area were higher under dynamic conditions (Fig. 8). This confirmed higher cell viability and growth under the dynamic conditions. The low cell viability and reduced cell growth under the static conditions can be related to the variation in pH and is discussed below. The expression level of vinculin (adhesion protein) and actin (skeletal protein) was higher under the dynamic conditions (Fig. 9). Vinculin is involved in the linkage of adhesion receptors with the cytoskeleton and is responsible for cell–cell and cell–matrix binding. Also, vinculin contributes to the coordination of cellular signaling along with the transduction of mechanical force to the cytoskeleton.37,38 Apart from this, actin plays an important role in cellular functions such as cell division, cell signaling, cell motility, cell polarity, and control of cell morphology.39,40 Also, the increased turnover of actin filaments increases cell life, while decreased throughput of actin may trigger the cell death through apoptosis.41 Thus, higher expression of actin and vinculin is an indication of strong cell–cell and cell–matrix binding as well as cell longevity and enhanced cell signaling, required for increased proliferation of osteoblast. Overall, dynamic conditions were favorable for cell proliferation in the presence of an electric field.
Under static conditions, the pH measurement indicated a small decrease in pH (∼7) near the anode when the applied electric field was 1 V cm−1, which was close to the physiological pH (∼7.4). However, in the vicinity of the cathode (∼8.5) and in the mid-region (∼8), a higher pH was measured. Interestingly, as compared to the anode, a higher cell density was found in the cathode region with alkaline pH. Moreover, the cell-inhibition zone in the vicinity of the cathode was observed to be associated with H2 gas evolution. But the evolution of H2 gas was less under the dynamic conditions, which led to smaller cell-inhibition zone. In this case, no effect of pH was considered because the old media was continuously replaced with the new media.
Another aspect of interest is the confluency of cells because confluency of cells affects cell migration under the effect of the electric field.42,43 The sparse cell motility increases with the increase in electric field strength.7 Furthermore, the reason behind the differential effect of the electric field on sparse and monolayer cell viability is that in sparse cells, a smaller voltage drop occurs across individual cells, while in the monolayer, voltage drop occurs across the several contacting cells, which communicate via gap junction.44,45 A higher voltage drop occurs at higher electric field. Under these conditions the membrane permeability reaches to a level that it may require a few hours for the cells to recover, referred to as reversible breakdown, while cell death may occur in irreversible breakdown. Thus, under the static conditions, a higher damage to the cells in the monolayer (confluent) was observed than sparse cells (not confluent).
On the basis of aforementioned discussion, it is obvious that the cellular activity can be tailored using an external electric field under the dynamic conditions. We believe that in the presence of flow of media and 1 V cm−1 electric field, the enhanced synthesis of vinculin and actin polymerization followed by extracellular and intracellular signaling is the mechanism behind higher cell proliferation and cell longevity under dynamic conditions as compared to the static conditions. Importantly, the electric field stimulation under static conditions does not mimic the physiological conditions because naturally blood flow maintains the tissue homeostasis, in vivo.
To further extend our understanding on the effect of media flow on cell behavior in the presence of an electric field and to simulate the conditions of blood flow in arteries and capillaries, we are currently studying porous scaffolds. This will help us design suitable porous scaffolds for tissue engineering applications that can be stimulated/activated using an external electric field for rapid tissue regeneration.
5. Conclusions
The concept of electric field mediated growth of cells for faster wound healing was studied under static and dynamic conditions. The pulsed DC electric field of 1 V cm−1 strength had a differential effect on monolayer and sparse osteoblasts. In the presence of an electric field the osteoblasts migrated towards the cathode in the direction parallel to the applied electric field such that lamellipodia was aligned. This cell alignment was more prominent under the dynamic conditions.Under static conditions, 1 V cm−1 electric field led to strong detrimental effect on sparse osteoblasts, characterized by cell blebbing and disruption of actin filaments. This led to cell apoptosis and decreased proliferation. In contrast, under the dynamic conditions, enhanced turnover and polymerization of actin filaments and presence of bundle of stress actin fibers in the vicinity of electrodes and mid-region led to significant proliferation in comparison to the static conditions. In comparison to control, higher turnover of actin and a thick bundle of actin stress fibers were observed under the dynamic conditions in the presence of a pulsed electric field of 1 V cm−1.
References
- P. Boscolo, M. Di Gioacchino, L. Di Giampaolo, A. Antonucci and S. Di Luzio, Int. J. Immunopathol. Pharmacol., 2007, 20, 59–63 CAS.
- M. E. Mycielska and M. B. A. Djamgoz, J. Cell Sci., 2004, 117, 1631–1639 CrossRef CAS PubMed.
- B. Song, M. Zhao, J. Forrester and C. McCaig, J. Cell Sci., 2004, 117, 4681–4690 CrossRef CAS PubMed.
- I. Titushkin and M. Cho, Biophys. J., 2009, 96, 717–728 CrossRef CAS PubMed.
- M. R. Cho, H. S. Thatte, R. C. Lee and D. E. Golan, FASEB J., 1996, 10, 1552–1558 CAS.
- X. Li and J. Kolega, J. Vasc. Res., 2002, 39, 391–404 CrossRef CAS PubMed.
- E. Finkelstein, W. Chang, P. H. G. Chao, D. Gruber, A. Minden, C. T. Hung and J. C. Bulinski, J. Cell Sci., 2004, 117, 1533–1545 CrossRef CAS PubMed.
- C. D. McCaig, L. Sangster and R. Stewart, Dev. Dyn., 2000, 217, 299–308 CrossRef CAS.
- H. Sauer, G. Rahimi, J. Hescheler and M. Wartenberg, J. Cell. Biochem., 1999, 75, 710–723 CrossRef CAS.
- S. Sun, Y. Liu, S. Lipsky and M. Cho, FASEB J., 2007, 21, 1472–1480 CrossRef CAS PubMed.
- D. M. Ciombor and R. K. Aaron, Foot Ankle Clin., 2005, 10, 579–593 CrossRef PubMed.
- G. A. Gordon, J. Cell. Physiol., 2007, 212, 579–582 CrossRef CAS PubMed.
- D. Janigro, C. Perju, V. Fazio, K. Hallene, G. Dini, M. K. Agarwal and L. Cucullo, BMC Cancer, 2006, 6, 72 CrossRef PubMed.
- M. S. Markov, Electromagn. Biol. Med., 2007, 26, 257–274 CrossRef PubMed.
- B. F. Sisken, J. Walker and M. Orgel, J. Cell. Biochem., 1993, 51, 404–409 CrossRef CAS PubMed.
- I. Titushkin and M. Cho, Biophys. J., 2006, 90, 2582–2591 CrossRef CAS PubMed.
- P. S. Hair, K. H. Schoenbach and E. S. Buescher, Bioelectrochemistry, 2003, 61, 65–72 CrossRef CAS.
- K. H. Schoenbach, S. J. Beebe and E. S. Buescher, Bioelectromagnetics, 2001, 22, 440–448 CrossRef CAS PubMed.
- S. J. Beebe, P. M. Fox, L. J. Rec, K. Somers, R. H. Stark and K. H. Schoenbach, IEEE Trans. Plasma Sci., 2002, 30, 286–292 CrossRef CAS.
- K. H. Schoenbach, F. E. Peterkin, R. W. Alden Iii and S. J. Beebe, IEEE Trans. Plasma Sci., 1997, 25, 284–292 CrossRef.
- A. K. Dubey, S. D. Gupta and B. Basu, J. Biomed. Mater. Res., Part B, 2011, 98, 18–29 CrossRef PubMed.
- B. Ercan and T. J. Webster, Int. J. Nanomed., 2008, 3, 477 CAS.
- C. A. Erickson and R. Nuccitelli, J. Cell Biol., 1984, 98, 296–307 CrossRef CAS.
- G. Fuhr, H. Glasser, T. Müller and T. Schnelle, Biochim. Biophys. Acta, Gen. Subj., 1994, 1201, 353–360 CrossRef CAS.
- A. Kotwal and C. E. Schmidt, Biomaterials, 2001, 22, 1055–1064 CrossRef CAS.
- K. H. Schoenbach, R. P. Joshi, J. F. Kolb, N. Chen, M. Stacey, P. F. Blackmore, E. S. Buescher and S. J. Beebe, Proc. IEEE, 2004, 92, 1122–1137 CrossRef CAS.
- R. A. Hoffman and W. B. Britt, J. Histochem. Cytochem., 1979, 27, 234–240 CrossRef CAS PubMed.
- C. A. Bassett, S. N. Mitchell, L. Norton and A. A. Pilla, Acta Orthop. Belg., 1978, 44, 706 CAS.
- C. Brighton, Z. B. Friedenberg, E. Mitchell and R. Booth, Clin. Orthop. Relat. Res., 1977, 124, 106–123 Search PubMed.
- R. B. Heppenstall, Clin. Orthop. Relat. Res., 1983, 178, 179–184 Search PubMed.
- R. B. Borgens, L. F. Jaffe and M. J. Cohen, Proc. Natl. Acad. Sci. U. S. A., 1980, 77, 1209–1213 CrossRef CAS.
- R. B. Borgens, E. Roederer and M. J. Cohen, Science, 1981, 213, 611–617 CAS.
- I. Titushkin and M. Cho, Biophys. J., 2007, 93, 3693–3702 CrossRef CAS PubMed.
- J. Chen and M. C. Wagner, Am. J. Physiol.: Renal, Fluid Electrolyte Physiol., 2001, 280, F619–F627 CAS.
- L. Vodovnik, D. Miklavčič and G. Serša, Med. Biol. Eng. Comput., 1992, 30, CE21–CE28 CAS.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 2007 Search PubMed.
- K. A. DeMali, Trends Biochem. Sci., 2004, 29, 565–567 CrossRef CAS PubMed.
- A. Carisey and C. Ballestrem, Eur. J. Cell Biol., 2011, 90, 157–163 CrossRef CAS PubMed.
- R. Dominguez and K. C. Holmes, Annu. Rev. Biophys., 2011, 40, 169 CrossRef CAS PubMed.
- H. F. Lodish, A. Berk, S. L. Zipursky, P. Matsudaira, D. Baltimore and J. Darnell, Molecular Cell Biology, W. H. Freeman, New York, USA, 4th edn, 2000, pp. 1150 Search PubMed.
- C. W. Gourlay and K. R. Ayscough, Nat. Rev. Mol. Cell. Biol., 2005, 6, 583–589 CrossRef CAS PubMed.
- M. Abercrombie, Eur. J. Cancer (1965–1981), 1970, 6, 7–13 CrossRef CAS.
- M. Abercrombie, Proc. R. Soc. London, Ser. B, 1977, 199, 337–344 CrossRef CAS.
- R. Azarnia and T. R. Russell, J. Cell Biol., 1985, 100, 265–269 CrossRef CAS.
- T. Saito, R. Schlegel, T. Andresson, L. Yuge, M. Yamamoto and H. Yamasaki, Oncogene, 1998, 17, 1673–1680 CAS.
| This journal is © The Royal Society of Chemistry 2016 |