Development and validation of an UHPLC-MS/MS method for the simultaneous determination of four flavonoid glycosides and phellopterin in rat plasma: application to a pharmacokinetic study of oral administration of Poncirus trifoliata extract
Abstract
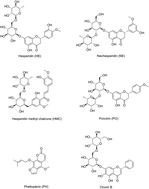
A simple, sensitive and selective ultra high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was developed for the simultaneous determination and pharmacokinetic study of five active components, hesperidin, neohesperidin, hesperidin methyl chalcone, poncirin, and phellopterin in rat plasma after oral administration of Poncirus trifoliata extract for the first time. This method involves a simple protein precipitation extraction with acetonitrile. The separation was performed on an Agilent XDB C18 column (100 × 2.1 mm and 1.8 μm) by gradient elution using a mobile phase composed of acetonitrile and water containing 0.1% formic acid at a flow rate of 0.35 mL min−1. Selective reaction monitoring was used for the quantification of the five active components and an internal standard (IS, oroxin B). The method was linear for all analytes over their investigated ranges with all correlation coefficients greater than 0.995. The lower limits of quantification (LLOQ) were 2.0 ng mL−1 for hesperidin, neohesperidin, hesperidin methyl chalcone, and poncirin, and 1.0 ng mL−1 for phellopterin. Intra- and inter-day precisions (RSD%) were in the range of 0.8–12.9% and the accuracies were between 87.2 and 108.3%. The developed method was successfully applied to the pharmacokinetic study of hesperidin, neohesperidin, hesperidin methyl chalcone, poncirin, and phellopterin in rat plasma after oral administration of Poncirus trifoliata extract.

 Please wait while we load your content...
Please wait while we load your content...