Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved strategies for selection and characterization of new monoclonal anti-carbamazepine antibodies during the screening process using feces and fluorescence polarization immunoassay†
Lidia
Oberleitner
ab,
Ursula
Dahmen-Levison
c,
Leif-Alexander
Garbe
b and
Rudolf J.
Schneider
 *a
*a
aBundesanstalt für Materialforschung und -prüfung (BAM), 12205 Berlin, Germany. E-mail: rudolf.schneider@bam.de; Fax: +49 30 8104 71151; Tel: +49 30 8104 1151
bInstitute of Bioanalytics, Department of Biotechnology, Technische Universität Berlin, 13353 Berlin, Germany
caokin AG, Robert-Rössle-Str. 10, 13125 Berlin, Germany
First published on 18th August 2016
Abstract
Immunoassays are suitable tools for high-throughput screenings. The prerequisite for accurate determinations by these methods is the selection of an excellent antibody. The production and selection of monoclonal antibodies is usually a tedious process. In this study, new strategies for improving antibody production and characterization were applied. This includes the monitoring of the immunization progress in mice through antibodies extracted from feces, which allows a time-resolved and animal-friendly monitoring of the immune response. Additionally, fluorescence polarization immunoassay (FPIA) could be successfully applied for fast and easy examination of cell culture supernatants and the investigation of antibody/antigen interactions including kinetics and fluorescence properties. These methods simplify the selection of the optimal antibody. As a target analyte, carbamazepine was chosen. This is a widely used antiepileptic drug which also frequently occurs in the environment. The new antibody enables CBZ determination in the concentration range 0.66–110 μg L−1 within 10 min using a high-throughput microtiter plate-based FPIA, and between 1.4 and 79 μg L−1 within 5 min applying an automated cuvette-based FPIA instrument, and from 0.05–36 μg L−1 using ELISA. The measurements were performed at a non-equilibrium state which improved the sensitivity and selectivity of the assays. Due to low cross-reactivity especially towards the main CBZ metabolite and other pharmaceuticals (<1%), this antibody gives the opportunity for application in medical and environmental analyses.
Introduction
Immunoassays are bioanalytical methods that are based on the high specificity of the binding between an antibody and its antigen. Usually these methods are performed on 96-well microtiter plates (MTPs) and thus they are characterized by a very high throughput. The most commonly used immunoassay is the enzyme-linked immunosorbent assay (ELISA), which belongs to the group of heterogeneous assays and shows very high sensitivity. But this assay includes long incubation steps (0.5–2 h), an overnight coating step and several washing steps. The fluorescence polarization immunoassay (FPIA) represents a fast alternative. This assay belongs to the group of homogeneous assays, which means that no washing steps are required. Additionally, FPIA usually only requires one short incubation step of a few minutes. The principle of this assay has been described in detail many times.1,2The prerequisite for a sensitive and accurate determination with immunoassays is the availability of a highly selective antibody with high affinity to the target analyte. Therefore, a thorough characterization of new antibodies is required.
The common protocol for the production of monoclonal antibodies starts with the immunization of one or more mice. The blood of the mice is examined by ELISA to check the presence of anti-analyte specific antibodies. This practice is painful for the animals because usually the blood sample is taken by facial vein puncture, retrobulbary puncture or tail vein puncture. Tail loss and fatalities are not rare. In order to warrant good animal welfare, this test can only be performed at long time intervals. This makes it impossible to find the best moment for re-immunization or the termination of the immunization process. A more time-resolved method is therefore desirable. Carvalho et al. showed that the extraction and evaluation of antibodies from mouse feces is a good alternative to serum screening.3 Additionally, this allows a time-resolved evaluation of the immunization progress without hurting the animals. After several boosts, the mouse presenting the highest level of anti-analyte antibodies is selected for further steps of antibody production.
Next, spleen cells from the selected mouse are fused with myeloma cells as described by Köhler and Milstein.4 After the fusion, the cell culture supernatants have to be tested in order to decide which hybridoma cells are producing the best antibody. This screening is usually performed by ELISA, which is very time-consuming due to long incubation times. As a fast alternative, FPIA could be used as the screening method. The applicability of FPIA to antibody-enriched medium has been already shown by Kolosova et al.5 Additionally, FPIA can be used for the characterization of antibody properties.6
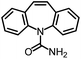
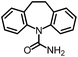
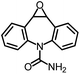
As the target analyte, carbamazepine (CBZ) was chosen. This is an antiepileptic drug, which is widely used in the treatment of trigeminal neuralgia, and grand mal seizures. It can also be used for the treatment of psychiatric disorders, e.g. bipolar disorder or borderline personality disorder.7 The main metabolic pathways of CBZ in humans and the distribution of extracted CBZ were summarized by Bahlmann et al.8 During human metabolism, mainly 10,11-epoxy-CBZ (Ep-CBZ) is formed. This intermediate is then enzymatically hydrolysed to 10,11-dihydro-10,11-dihydroxy-CBZ (DiOH-CBZ), which represents the major part of excreted CBZ.
Due to the widespread use and a low degradation rate in wastewater treatment plants, CBZ is often used as a marker for wastewater input into surface and groundwater.9,10 When CBZ enters surface water, it can reveal negative effects on the health status of aquatic organisms.11,12 Additional treatment steps like ozonation would improve the degradation rate in wastewater treatment plants.13,14 Therefore, CBZ can be seen as a marker for the elimination efficiency of wastewater treatment plants.14
ELISAs have been developed for CBZ and have been successfully applied to water samples.15,16 FPIA for the determination of CBZ in serum is already used17 and the application of this kind of assay to surface water samples has been recently described.18 Previously described CBZ immunoassays were performed with a monoclonal anti-CBZ antibody, which showed high cross-reactivity (CR) against CBZ metabolites and related compounds, but also to the antihistaminic drug cetirizine which is not structurally close to CBZ.19,20 This leads to overestimations of CBZ levels in water samples, especially during the hay fever season, when the antihistamine is present in waters. To avoid this effect, a new, more selective monoclonal antibody against CBZ was desirable.
The goal of this work was to manage to produce a new highly specific monoclonal anti-CBZ antibody via improved strategies using animal-friendly and time-efficient methods for the monitoring of the immunization progress, the antibody selection and the antibody characterization.
Materials and methods
Reagents and materials
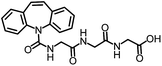
All solvents and chemicals were purchased from Sigma-Aldrich, Merck KGaA, Serva, Mallinckrodt Baker and Toronto Research Chemicals Inc. in the highest available quality. The FP tracer CBZ-triglycine-5-(aminoacetamido)fluorescein (CBZ-AAF) was previously synthesized.18 CBZ-triglycine and the tracer for ELISA, CBZ-triglycine-horseradish peroxidase (CBZ-HRP) were previously prepared by Bahlmann et al.15 CBZ-triglycine-ovalbumin (CBZ-OVA) was prepared following the same procedure.15 For the preparation of buffers and solutions, ultrapure water from a Milli-Q® Reference water purification system from Millipore was used. The compositions of the phosphate buffered saline (PBS) buffer, PBS-based washing buffer, sample buffer, citrate buffer, and 3,3′,5,5′-tetramethylbenzidine (TMB) solution were described previously.21During synthesis of the immunogen, a thermomixer compact (Eppendorf) was used. PD-10 desalting columns (GE Healthcare) were used for the purification of the immunogen. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) measurements using a Bruker Reflex III instrument (Bruker Daltonik) were carried out to determine the coupling ratio of the immunogen. 96-well clear UV-Star MTPs (Greiner Bio-One) were used for fractionating the synthesized immunogen. Clear high-binding and black non-binding 96-well MTPs from Greiner Bio-One were employed for ELISA and FP measurements, respectively. All assay incubation and shaking steps were performed on a plate shaker Titramax 101 from Heidolph (750 rpm). The MTPs for ELISA were washed using an automated plate washer from BioTek. For the measurements of absorbance (ELISA) and fluorescence polarization (FPIA), eon and Synergy H1 plate readers from BioTek were used, respectively. Both were controlled by the software Gen5 (BioTek). FPIA in cuvettes was performed on a filter-based aokin spectrometer FP 470 (aokin AG). The system was controlled by the aokin software mycontrol™. The excitation wavelength was fixed at 470 nm, and the emission was measured at 520 nm. The fluorescence intensities at perpendicular and parallel polarizer settings were measured simultaneously and continuously (kinetic measurement). For automated measurements, an aokin liquid handling workstation (LHW), which can be connected to the spectrometer, was used.
Immunogen synthesis
The N-hydroxysuccinimide (NHS)/N,N′-dicyclohexylcarbodiimide (DCC) activated ester method was used for the synthesis of the immunogen CBZ-triglycine-bovine serum albumin (CBZ-BSA). For this, 6.8 μmol of the hapten CBZ-triglycine were dissolved in 50 μL dimethylformamide (DMF). Then 20 μL of NHS (46.5 g L−1 in DMF) and DCC solution (83.5 g L−1 in DMF) were added. The mixture was shaken for 18 h in a thermomixer at 22 °C and 700 rpm. Then the reaction mixture was centrifuged for 10 min at 20 °C and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, in order to separate the solution from the precipitate formed. BSA (6.0 mg) was dissolved in 600 μL of a 0.27 mol L−1 sodium hydrogen carbonate solution. Into that solution, small volumes of the activated ester solution were added every few minutes (12 × 5 μL). Between the pipetting steps, the reaction mixture was shaken in the thermomixer. After in total 60 μL of the activated ester was added to the BSA solution, the mixture was shaken for 4 more hours at 22 °C and 700 rpm.
000 rpm, in order to separate the solution from the precipitate formed. BSA (6.0 mg) was dissolved in 600 μL of a 0.27 mol L−1 sodium hydrogen carbonate solution. Into that solution, small volumes of the activated ester solution were added every few minutes (12 × 5 μL). Between the pipetting steps, the reaction mixture was shaken in the thermomixer. After in total 60 μL of the activated ester was added to the BSA solution, the mixture was shaken for 4 more hours at 22 °C and 700 rpm.
The conjugate was purified using a PD-10 desalting column. The column was first equilibrated with 25 mL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diluted PBS buffer (pH 7.6). Then the reaction mixture was applied to the column and was then eluted with 7.5 mL of the diluted PBS buffer. The fractions were collected in a MTP (three drops per well) and the absorbance was measured at 280 nm with a reference wavelength of 620 nm. The fractions with an optical density (OD) higher than 0.5 were collected.
10 diluted PBS buffer (pH 7.6). Then the reaction mixture was applied to the column and was then eluted with 7.5 mL of the diluted PBS buffer. The fractions were collected in a MTP (three drops per well) and the absorbance was measured at 280 nm with a reference wavelength of 620 nm. The fractions with an optical density (OD) higher than 0.5 were collected.
The collected fraction was applied on a Zeba™ spin micro-desalting column. A dihydroxyacetophenone (DHAP) matrix was used for MALDI-TOF-MS measurements. Masses of 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 454 and 76
454 and 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635 Da were determined for the BSA and CBZ-BSA conjugate, respectively. CBZ-triglycine minus water has a mass of 390 Da. Consequently, the mean coupling ratio was 26 molecules of CBZ-triglycine per BSA molecule. The protein concentration of the CBZ-BSA (3.2 g L−1) was determined using a Bradford assay as described before.21,22
635 Da were determined for the BSA and CBZ-BSA conjugate, respectively. CBZ-triglycine minus water has a mass of 390 Da. Consequently, the mean coupling ratio was 26 molecules of CBZ-triglycine per BSA molecule. The protein concentration of the CBZ-BSA (3.2 g L−1) was determined using a Bradford assay as described before.21,22
Antibody production
The production of the anti-CBZ antibodies including the immunization, fusion, cultivation, purification and subisotyping was performed at hybrotec GmbH (Potsdam, Germany). All animal experiments were conducted in accordance with animal ethical care regulations and with German law after approval of the experiments with live subjects by the respective committee, LUGV (Landesamt für Umwelt, Gesundheit und Verbraucherschutz), Abteilung Verbraucherschutz, Referat V3, Seeburger Chaussee 2, 14476 Potsdam. For the immunization of three Balb/c mice (mouse 1–3), the immunogen CBZ-BSA was used. For the first injection, 100 μg of the conjugate with Freund's adjuvant were used for each mouse. After 42 days, another 50 μg were injected. Blood samples were tested 48 days after the first injection. The mouse with the highest antibody titer, determined by indirect ELISA using CBZ-OVA, was chosen for the production of monoclonal anti-CBZ antibodies. Only a few CBZ calibrators were used to verify the recognition of free CBZ by the antibodies present in the serum samples. After another CBZ-BSA injection (day 112), spleen cells of this mouse (mouse 1) were fused with myeloma cells 116 days after the first immunization.The resulting hybridoma cells were cultivated in eight 96-well MTPs. The presence of anti-CBZ antibodies was tested for the supernatants of all these clones with an indirect, competitive ELISA. Therefore, ultrapure water was used as the “calibrator” to test for the presence of anti-hapten antibodies. Additionally, one calibrator with a high CBZ concentration was used to verify the binding of free CBZ by the antibody. 14 clones showed a reaction with CBZ-OVA and five of them gave a reasonably high signal. For these antibodies, a significant inhibition by free CBZ was observed. For further investigations, 0.1% sodium azide was added to the supernatants of the five selected clones. After additional testing, these clones were further cultivated and purified through a protein A column and the subclasses for each of these antibodies were determined (all subisotype IgG1).
The purified antibodies were stored at −20 °C after adding different amounts of glycerol, depending on the antibody concentration. Too low concentrations should be avoided. In general, 50% of glycerol were added, but due to lower concentrations of antibodies from clone 2 and 4, only 25% glycerol were added so that all concentrations were higher than 400 mg L−1 for long-term storage.
Feces screening
Feces samples of all three mice were collected from day 11 after the immunization and then every 7 days. The samples were stored at −20 °C until analysis. The antibodies from these samples were extracted by dissolving the feces in extraction buffer (1.5 mL extraction buffer per 0.1 g feces). The extraction buffer was prepared by dissolving 1% BSA, 1% NaN3 and 2 tablets protease inhibitor cocktail tablets (Roche) in 100 mL PBS buffer. The mixtures of extraction buffer and feces were shaken for 23 h in centrifugation tubes on a shaking table with 80 rpm at room temperature. Afterwards the mixtures were centrifuged two times for 10 min at room temperature. The supernatants were used to analyze the content of anti-CBZ specific antibodies using direct, competitive ELISA as described later on. Instead of monoclonal anti-CBZ antibodies, the undiluted feces extracts were used. When enough extract was present, a triplicate determination was performed. Unfortunately in some cases not enough feces could be collected and therefore not enough extract could be produced. For some samples, only a single or duplicate determination could be performed.Direct competitive ELISA
For direct, competitive ELISA, each well was coated with 200 μL anti-mouse IgG antibody (polyclonal, sheep, Cat.-no. R1256P, lot 21481, Acris Antibodies) at 1 mg L−1 in PBS buffer. MTPs were covered with Parafilm® M and shaken at 750 rpm for 18 h. The MTPs were then washed three times with an automatic plate washer using a PBS-based washing buffer.Then 200 μL of the respective anti-CBZ antibodies were added to each well and incubated for 1 h. For the feces screening, undiluted feces extracts instead of the monoclonal antibody dilutions were used. For investigations of cell culture supernatants, different dilutions of supernatants were used, so that the upper asymptote was comparable for all clones. The fully optimized assay described here uses the antibody from clone 1 diluted in PBS buffer at a concentration of 7.5 μg L−1.
After another washing step, 150 μL of different calibrators were added to the respective wells. For the comparison of the sensitivity of the antibodies, only CBZ calibrators were used. For the determination of CRs, calibrators of the different CBZ-related substances were used. Directly after adding the calibrators, 50 μL of the CBZ-HRP conjugate diluted in sample buffer (8.3 μg L−1, pH 9.5) were added. For feces screening and investigations on cell culture supernatants, a higher tracer concentration of 16.6 μg L−1 was chosen. After a 30 min incubation period and another washing step, the TMB substrate solution was added. This solution was prepared according to the following protocol:23 21 mL citrate buffer with 8.1 μL hydrogen peroxide (30%) and 525 μL TMB solution were mixed and 200 μL were added to each well. The reaction was stopped after 30 min by adding 100 μL of 1 mol L−1 sulfuric acid. Absorbance was measured at 450 nm and referenced to 620 nm.
The precision profile was determined by measuring 16 CBZ calibrators in sixtuplicate. For calculations, the software Origin 9.1G (OriginLab) was used. As described by Ekins, the relative errors of concentration were calculated.24 The concentrations with a relative error of lower than 30% were defined as the measurement range. This value was chosen following the three sigma criterion as described previously.21
FPIA on MTP
For the homogeneous assay on MTPs, 280 μL borate buffer (2.5 mmol L−1 disodium tetraborate decahydrate, 0.01% sodium azide, pH 8.5) with 0.01% Triton™ X-100 were pipetted into each well. After adding 20 μL of calibrators, a background measurement was performed with excitation at 485 nm and emission at 528 nm (using a polarizer at parallel and perpendicular settings). 20 μL of the tracer CBZ–AAF, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, diluted in a PBS-based tracer stabilization buffer,18 was added to each well and shaken for 5 min. Then 20 μL of anti-CBZ antibody in a PBS-based antibody stabilization buffer18 were added. For the final assay, 20 μL of the antibody from clone 1 (375 μg L−1) were used. After 10 min of shaking, the fluorescence intensities were measured with the settings described above.
000, diluted in a PBS-based tracer stabilization buffer,18 was added to each well and shaken for 5 min. Then 20 μL of anti-CBZ antibody in a PBS-based antibody stabilization buffer18 were added. For the final assay, 20 μL of the antibody from clone 1 (375 μg L−1) were used. After 10 min of shaking, the fluorescence intensities were measured with the settings described above.
The total fluorescence intensities were determined as the sum of the parallel and double perpendicular intensity. The G factor was set to 1.0. The fluorescence intensities at perpendicular and parallel polarizer settings from the background measurement were subtracted from the respective values. These background-corrected values were then used for the calculation of the degree of polarization. The precision profile for clone 1 was determined as described for ELISA.
FPIA in cuvettes
For the examination of the cell culture supernatants with FPIA in cuvettes, all steps were performed manually. First, 2 mL borate buffer were pipetted into a round glass cuvette containing a stir bar. 100 μL ultrapure water was added instead of calibrators. Then 100 μL of tracer dilution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in stabilization buffer) were added. Afterwards, small volumes of the cell culture supernatants were pipetted into the cuvette. The degrees of polarization were corrected by the background signals and the G factor (determined for each measurement) and the degree of polarization of the free tracer was subtracted.
000 in stabilization buffer) were added. Afterwards, small volumes of the cell culture supernatants were pipetted into the cuvette. The degrees of polarization were corrected by the background signals and the G factor (determined for each measurement) and the degree of polarization of the free tracer was subtracted.
The calibration curve and precision profile of the selected antibody (clone 1) were determined automatically using the LHW. All volumes were adapted from the manual measurement described above, besides the calibrator (here: 200 μL) and the antibody. Here, 100 μL of a dilution of the purified antibodies of clone 1 (1500 μg L−1) in stabilization buffer were used. All calibrators were measured in triplicate. The G factor was fixed at 1.10.
Cross-reactivity
The CR of twelve substances was determined with ELISA and FPIA on MTPs: 10,11-dihydro-CBZ (DiH-CBZ), Ep-CBZ, oxcarbazepine (Ox-CBZ), DiOH-CBZ, 10,11-dihydro-10-hydroxy-CBZ (10-OH-CBZ), 2-hydroxy-CBZ (2-OH-CBZ), 3-hydroxy-CBZ (3-OH-CBZ), CBZ-triglycine, iminostilbene, opipramol dihydrochloride, loratadine and cetirizine (CET). Each cross-reactant was determined in triplicate on each MTP and on two MTPs. The molar CRs were determined dividing the molar test midpoint of CBZ by the molar test midpoint of the cross-reactant. The CR towards 2-OH-, 3-OH- and DiH-CBZ was additionally determined on one MTP for ELISA at pH 8.5 (pH of the sample buffer was varied). The CR of DiOH-CBZ, 2-OH-CBZ and CET was also determined on one MTP for cell culture supernatants from clone 1–5 using ELISA.Results and discussion
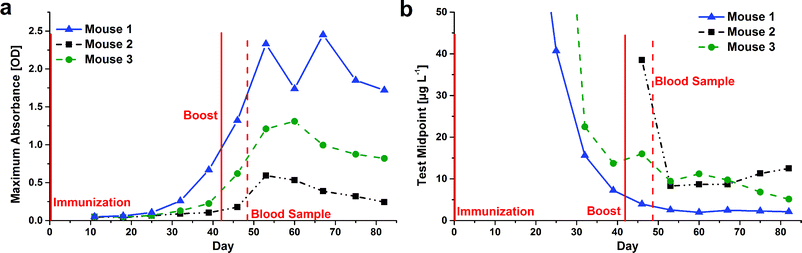
Immunization progress via feces testing
Usually, the production of analyte-specific antibodies in mice is monitored by collecting and analyzing blood samples. This painful procedure can be replaced by analysis of feces, in which antibodies can also be found. Additionally, this kind of samples can be collected more frequently than blood samples.The feces from all three immunized mice were collected every week and antibodies from mice feces were extracted. With the undiluted extracts, calibration curves were set up with direct, competitive ELISA. The maximum absorbance as a measure for the antibody titer and the test midpoint as an indicator for affinity were determined (Fig. 1). The immune response of the three mice differed considerably. After the immunization, nearly no signal could be detected for mouse 2, i.e. almost no anti-CBZ antibodies were found in the feces of this mouse; i.e. this mouse did not produce anti-CBZ antibodies until the first boost. In feces of mice 1 and 3 an increasing antibody titer was observed even before the first boost (Fig. 1a). Moreover the affinity increased strongly (lower test midpoints) before the second dose of the immunogen was administered to the mice (Fig. 1b).
The maximum absorbances for all three mice decreased after having reached their maxima after the first boost. But the test midpoints stayed almost constant at their lowest levels. So the reached affinity seems not to deteriorate again, even when there is no new contact with the immunogen for a while.
For the blood samples collected 48 days after the immunization, the maximum absorbance of different dilutions was determined with indirect ELISA (performed at hybrotec). Blood from mouse 2 showed also the by far lowest absorbance for all dilution factors. Mouse 1 and 3 showed values in a similar range, the results for mouse 1 being a little bit better. To get an estimate of the binding strength of the antibodies from the sera for free CBZ, a few CBZ calibrators were used during these experiments. For all blood samples, recognition of free CBZ by the antibodies from sera was observed.
The tendencies of the results from blood and feces screening were in accordance with each other, while much more information can be obtained using the feces method and this without hurting the animals. A direct comparison of both methods is not possible because two different methods were used (direct and indirect ELISA) and the antibody concentrations in blood samples are usually much higher than those in feces extracts.
In a previous study, only the comparability of feces and blood sampling on one specific day was studied.3 Here, it could be shown that also the development of antibodies in the course of time can be monitored by this method. Mouse 1 was finally selected for spleen removal and fusion of B-cells with myeloma cells.
Characterization of antibodies in cell culture supernatants by FPIA
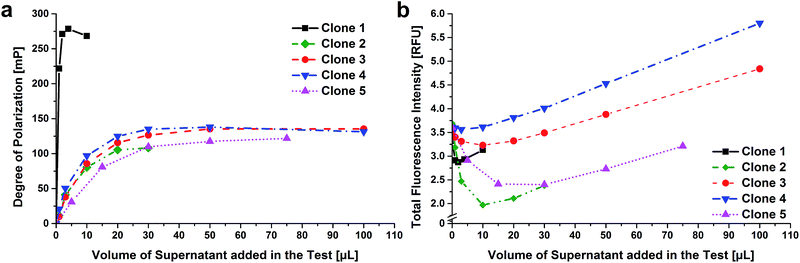
The supernatants of hybridoma cells (8 × 96) were tested with an indirect ELISA (performed at hybrotec) and the five best clones, showing the highest signals, were selected. All these clones showed also an inhibition by CBZ. The properties of these antibodies in cell culture supernatants were investigated with FPIA. Therefore the assay was performed in cuvettes with an instrument which allows the kinetic observation of the tracer/antibody reaction.25 Different buffers were tested to select the optimum conditions for FPIA measurements with the selected cell culture supernatants: carbonate buffer pH 9.6, sample buffer pH 9.5, Tris buffer pH 8.5, borate buffer pH 8.5 and PBS buffer pH 7.6. Only buffers with neutral to alkaline pH values were selected because the fluorescence intensity of the fluorescein tracer decreases considerably under acidic conditions. For all clones, besides clone 2, borate buffer led to the highest degree of polarization values using the smallest volume of the supernatant. For clone 2, carbonate buffer led to the best results. For a better comparability, borate buffer was used for further experiments.The maximum degrees of polarization (Pmax) for different supernatants were determined by adding continuously small amounts of supernatant to the buffer containing the CBZ–fluorescein tracer (Fig. 2a). For clone 1, Pmax was already reached after adding 2 μL of the supernatant. For the other clones, Pmax was not reached until 20 μL (clone 2) or 30 μL (clone 3–5) of supernatant had been added. Pmax of clone 1 with 280 mP was much higher compared to that of all other supernatants (100–140 mP). So the by far highest affinity towards the tracer was observed for the antibodies in the supernatant of clone 1.
The supernatants showed an intense color due to phenol red that is contained in the cell culture medium used. Therefore it was expected that the fluorescence intensity would increase the more supernatant was added to the assay. This was the case for clones 1, 3 and 4, but not for clones 2 and 5 (Fig. 2b). Here, a first strong decrease of the fluorescence intensities was observed before the intensities increased again. This means that the antibodies in the supernatants significantly reduced the fluorescence intensity of the tracer. After adding a certain volume of the supernatant, the fluorescence intensities increased again due to the high amount of phenol red. So for purified antibodies, it is expected that the fluorescence intensity does not increase again. This can have negative effects on FPIA performance because the measured values are fluorescence intensities and based on them the degree of polarization is determined. So if the measured intensities are low, the relative error increases and therefore also the error of the determined degree of polarization becomes larger.
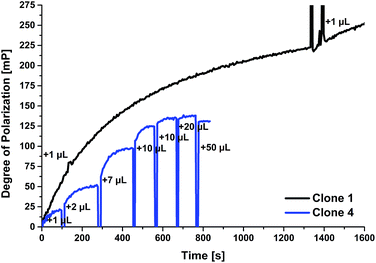
Another interesting issue is the kinetics of the tracer/antibody interaction. Usually this reaction is finished within a few hundred seconds, e.g. for a previously described caffeine FPIA using the same instrument, the equilibrium was reached after 100 s.25 Here, similar reaction times were observed: 100 s for clones 3 and 5, and 200 s for clones 2 and 4 (exemplarily shown for clone 4 in Fig. 3). Antibodies from clone 1 showed a much slower reaction with the tracer (1400 s, Fig. 3). But much less supernatant is necessary to reach a much higher degree of polarization than for all other supernatants. Using FPIA, a lot of information about the properties of the antibody, e.g. influences on the fluorescence of the tracer like quenching or enhancement, and the kinetics of the antigen/antibody interaction can be obtained already at this stage of antibody production. Therefore the implementation of this fast method for cell culture supernatant screening can simplify the selection of the antibodies with optimal properties for the desired application.
CR of the antibodies in cell culture supernatants was determined by direct, competitive ELISA for some selected cross-reactants. DiOH-CBZ is the main metabolite of CBZ and is therefore frequently found in wastewater in high concentrations. Compared to the structure of CBZ, this substance shows a change in the central, nitrogen-containing ring. To investigate the influence of changes of other parts of CBZ, CR against 2-OH-CBZ was determined. 4.3% of CBZ are excreted as 2-OH-CBZ.8 Cetirizine was chosen because it was one of the main cross-reactants of the previously used monoclonal anti-CBZ antibody, although CET is not structurally closely related to CBZ.19,20
Here, only semi-quantitative statements can be made, because these results were only produced to simplify the choice of the right antibody. All antibodies (from supernatants) showed very low CR (<1%) against DiOH-CBZ and CET. For the latter, antibodies from clone 5 showed a higher CR of approximately 8%. This is still a much lower CR than the one the previously used antibody showed towards this pharmaceutical.19 Nevertheless this would lead to an overestimation of CBZ determination in water samples. For 2-OH-CBZ, comparable CRs were observed for all antibodies (10–15%) except clone 2 (ca. 45%). It is noticeable that, with two exceptions, all CRs of the antibodies were similar for at least the three tested cross-reactants.
Characterization and comparison of purified antibodies
After the purification of the selected antibodies, the best antibody was to be carefully chosen. The FPIA on MTPs was used for this evaluation because more measurements can be performed simultaneously. First, different amounts of antibody (constant volumes of antibody dilutions were used with different dilution factors) were added to a constant amount of tracer in order to determine Pmax (ESI, Fig. S1a†). Clone 1 showed the by far highest Pmax (285 mP) and the lowest amount of antibody had to be employed (160 ng) to reach this level. This Pmax is in good agreement with the value obtained before for the antibodies contained in the hybridoma supernatants. For the other antibodies, higher Pmax values were obtained compared to the ones obtained for the respective supernatants (150–220 mP). For some antibodies, Pmax was not completely reached using 1000 ng antibody per measurement.Total fluorescence intensities decreased for all antibodies after the addition of the antibody doses (ESI Fig. S1b†). The fluorescence intensities showed only slight decreases when clone 3 (29%), 1 (22%) or 4 (21%) was used. However, the antibodies from clones 2 and 5 led to significant decreases of fluorescence intensity of 69 and 68%, respectively. The contrary effect was observed by Tan et al.26 They found that the binding of the tracer to the antibody increased the fluorescence intensity of the tracer. They used this effect and developed a homogeneous increasing fluorescence immunoassay (HiFi). They suggested that the fluorescence of fluorescein is quenched due to the coupled analyte (tetrahydrocannabinol). When the analyte part of the tracer is obscured due to the binding to the antibody, the quenching effect is eliminated and the fluorescence intensity increases. In our study, the opposite effect was observed. That means that the fluorescence of fluorescein is not quenched due to the coupled analyte. But the interaction with the antibody quenches the fluorescence intensity of the tracer. A reason could be that the conformation of CBZ is changed by the binding to the antibody. Eisold et al. observed both effects:27 two antibodies were compared that were produced in the same immunization process against a fluorophore: one antibody enhanced and the other antibody quenched the fluorescence intensity of the fluorophore. The idea of developing a homogeneous decreasing fluorescence immunoassay using clone 2 or 5 was not pursued in this study because first experiments with CBZ calibrators showed that the sensitivity of this assay would be quite low.
The results obtained for the purified antibodies are in good agreement with the assumptions made after the initial examination of the supernatants. As described above, a too strong decrease of the measured values would increase the measurement uncertainty. The tracer concentration could be increased to compensate the effect observed for clones 2 and 5. But this would lead to a reduction of the assay sensitivity. Additionally, first studies on CR performed with the supernatants showed higher non-specific binding for these antibodies. Calibration curves determined for ELISA confirmed that these two antibodies lead to less sensitive methods for the determination of CBZ than the other antibodies. Taking all this together into account, these two antibodies are not suitable for the development of a CBZ FPIA and therefore were not taken into consideration for further evaluation.
The studies on the cell culture supernatants already showed that the reaction times of the antibodies with the tracer vary considerably for different antibodies, especially for clone 1, where it took very long to reach the equilibrium. All other supernatants showed a quite fast reaction. This could be confirmed for purified antibodies using FPIA: for assays on MTPs, the reactions were finished within 5 min for clones 3 and 4, whereas clone 1 did not reach equilibrium before 30 min incubation time. For the standard assay procedure on MTPs, 10 min was chosen as the incubation time because it was well reproducible for the procedure of FPIA on MTPs even so as requiring shaking and transfer to the multimode plate reader. Additionally, a longer incubation time would be counterproductive with regard to one of the main advantages of FPIA: the quickness.
Calibration curves for the three remaining antibodies were determined on MTPs. For this the same amount of tracer was used and the antibody concentrations were optimized so that the dynamic range (the distance between upper and lower asymptotes of the calibration curve) was in a similar range of 130 ± 10 mP (ESI Fig. S2†). Under these conditions, a good comparability of the curves could be ensured. It should be mentioned that for clone 3 the highest amount of antibody had to be used per measurement (200 ng) to reach the desired dynamic range. Using clone 1, less than one tenth of the amount used of clone 4 was necessary to reach the desired dynamic range (7.5 instead of 86 ng per measurement, respectively). The assay using antibodies from clone 1 showed the best sensitivity with a test midpoint of 7.93 μg L−1, whereas clones 3 and 4 showed similar test midpoints of 170 and 137 μg L−1, respectively. With regard to sensitivity and the usually most expensive reagent of FPIA, the antibody, clone 1 was chosen for further antibody production and development of FPIA applications.
In addition to the careful examination for their use in FPIA, the antibodies from our clones were compared for their employment in direct competitive ELISA. Again the antibody from clone 1 showed the lowest test midpoint and therefore the highest measurement sensitivity. Consequently, this antibody is our choice also for its application in ELISA.
Characterization of the selected antibody (clone 1)
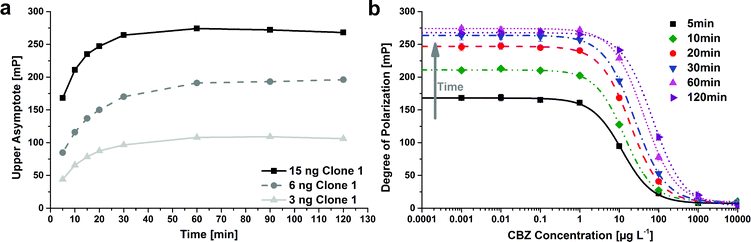
The calibration curve for one antibody dilution (15 ng purified antibody per measurement) was recorded after different times: 5, 10, 20, 30, 60, and 120 min (Fig. 4b). The upper asymptote increased from 168 mP to 274 mP. After 30 min incubation time, the upper asymptote did not increase any more whereas the test midpoint still increased after 30 min from 24 μg L−1, over 42 μg L−1 after 60 min, up to 58 μg L−1 after 120 min. The time-dependent increase of the test midpoints was also strong at shorter incubation times: the test midpoint increased from 12 μg L−1 after 5 min, to 13 μg L−1 after 10 min, up to 19 μg L−1 after 20 min. Therefore the compliance to the defined incubation time is very important. The effect of increasing test midpoint over incubation times was previously observed for ELISA for polyclonal28 and monoclonal antibodies, whereas the effect was stronger for polyclonals.29
The time dependency of the antibody reaction with the enzyme tracer was also studied by direct ELISA. Calibration curves were recorded after 15, 30, 45 and 60 min tracer incubation time. Here, the dynamic range increased from 0.35 up to 1.3 OD. The test midpoint varied only between 0.17 and 0.25 μg L−1, whereby there was no clear time dependency visible. To keep the assay time short, the incubation time of the ‘standard’ ELISA was kept (30 min).
The increase of the test midpoints for homogeneous assays, especially after the highest degree of polarization being reached, suggests that the antibody first reacts with the free analyte, which is then slowly replaced by the fluorophore tracer. For the heterogeneous assay the test midpoint does not continuously increase over time, i.e. the kinetics of antibody/tracer and antibody/analyte interactions are similar to each other. The interaction with both the analyte and enzyme tracer is slow, but none of them replaces the other due to a longer incubation time. For synthesis of fluorescein and enzyme tracers, respectively, the same hapten had been used. So the different kinetics towards the tracers may be induced by their different size (fluorescein tracer 795 Da, enzyme tracer 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 Da15). It could also be possible that the slightly higher ratio of hapten coupled to the enzyme of 1.5 ± 0.3
900 Da15). It could also be possible that the slightly higher ratio of hapten coupled to the enzyme of 1.5 ± 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 compared to 1
15 compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coupling of hapten and fluorescein is the reason for the different kinetics.
1 coupling of hapten and fluorescein is the reason for the different kinetics.
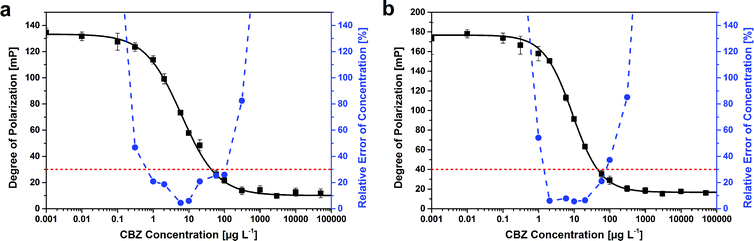
| Time [min] | Dynamic range [mP] | Slope | C [μg L−1] | R 2 | Measurement range [μg L−1] |
|---|---|---|---|---|---|
| 10 | 123 | 0.85 | 6.2 | 0.998 | 0.66–110 |
| 20 | 155 | 0.93 | 7.7 | 0.999 | 0.68–98 |
| 30 | 176 | 0.88 | 9.7 | 0.999 | 1.3–150 |
| 60 | 200 | 0.94 | 17 | 0.998 | 1.6–380 |
The highest standard deviation for each curve was very low with less than 8 mP. For a better comparability also to other immunoassays, the standard deviations of the degrees of polarization were normalized to the dynamic range. These normalized values decrease over time due to the increasing dynamic range. Nevertheless, the highest relative error was determined to be 5.2%.
In previous publications, quality criteria for the evaluation of immunoassays were defined including sensitivity, dynamic range, slope, goodness of fit and measurement range.16,21,25 Almost all these criteria were fulfilled for this assay at all incubation times, besides some slopes at the test midpoints; they should be 1.0 ± 0.1.25 The measurement ranges did also not reach the requirement of the width of three orders of magnitude that was stated for heterogeneous immunoassays.21 But it was already previously discussed that this value should be reduced for homogeneous assays.25 The measurement ranges determined after different incubation times reached all more than two orders of magnitude width, which is, compared to other FPIAs, rather good.
The tendencies over time of the different characteristic parameters (Table 2) are similar to those determined for FPIA on MTPs. Only the lower limit of the measurement range shows a different behavior: it does not increase so much. Here, the lower limit between the shortest and the longest incubation increased by 6%, whereas it increased by 140% for FPIA on MTPs. However, the upper limit of the measurement range shows a higher increase in cuvettes. The width of the measurement range reached almost three orders of magnitude after 30 min and is therefore similar to ELISA. In general, the FPIA on MTPs is slightly more sensitive and needs less antibody than the same assay performed in cuvettes.
| Time [min] | Dynamic range [mP] | Slope | C [μg L−1] | R 2 | Measurement range [μg L−1] |
|---|---|---|---|---|---|
| 5 | 160 | 1.1 | 8.9 | 1.00 | 1.4–79 |
| 10 | 221 | 1.0 | 11 | 1.00 | 1.4–290 |
| 15 | 249 | 1.0 | 13 | 1.00 | 1.5–210 |
| 30 | 273 | 1.0 | 21 | 1.00 | 1.5–1200 |
Besides the lowest CBZ calibrator, which showed a normalized standard deviation of 6.4–10%, all other errors were lower than 5.8% normalized to the dynamic range. Almost all characteristic parameters were in good agreement with the previously defined quality criteria.
ELISA is approximately 20 times more sensitive than the FPIA on MTPs regarding the test midpoint and the lower limit of the measurement range is 14 times lower. At the same time, 5 times as much antibody is used for FPIA on MTP (7.5 instead of 1.5 ng per measurement). Nevertheless, the performance of ELISA requires altogether 20 h, whereas for FPIA on MTPs the same amount of samples can be determined in 20 min, including all pipetting, incubation and measurement steps (incubation time of 10 min).
Differences between CRs of single cross-reactants determined by FPIA and competitive ELISA were reported previously. Kolosova et al. found that only CRs determined for a direct assay differ from results of FPIA, whereas the results from indirect ELISA were comparable with those of the homogeneous assay.30 However, Xu et al. also found different CRs using FPIA and indirect ELISA.31
CRs determined with ELISA using the cell culture supernatant and the purified antibody are in very good agreement: for CET and DiOH-CBZ for both types of antibodies of clone 1 CR was lower than 1%. The third tested substance, 2-OH-CBZ, showed a CR of 13% for the supernatant and 15% after purification of the antibody. So the results from supernatants can be attributed also to the purified antibody when the same assay type and assay conditions are used.
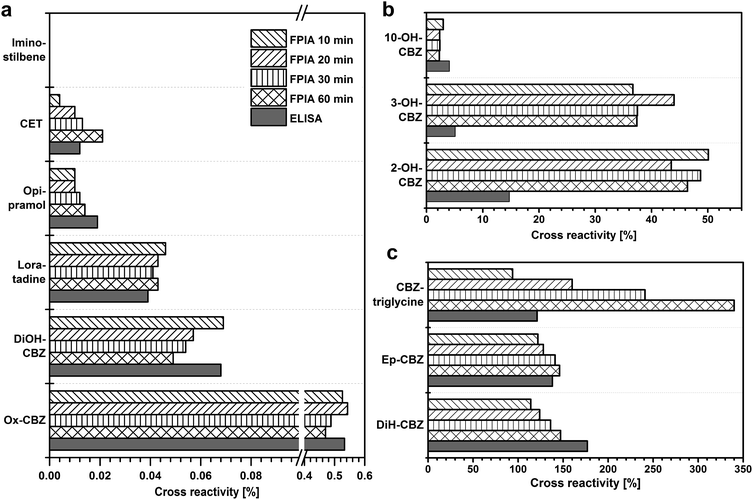
Due to the time dependency of the reaction noted for FPIA, the CR was additionally measured after 10, 20, 30 and 60 min incubation time (Fig. 6). For some cross-reactants, an increase of the CR was observed over time: CET (<0.01 to 0.21%), CBZ-triglycine (94 to 340%), Ep-CBZ (120 to 150%), and DiH-CBZ (110 to 150%). Therefore the strict compliance of the incubation time is very important, because a longer incubation time can lead to higher overestimations. This effect was previously observed for polyclonal antibodies: here, the CR increased with longer incubation times or remained stable.29 For two cross-reactants the antibody showed a decrease of the CR: DiOH-CBZ (from 0.07 to 0.05%) and Ox-CBZ (from 0.53 to 0.47%). But these differences are so small, that the benefit of a longer incubation time is negligible. For further considerations only the values after the standard FPIA incubation time of 10 min are taken into account.
After passing the human body, the highest amount of CBZ is excreted as DiOH-CBZ (32%).8 This is the main metabolite of CBZ, what makes it very valuable that the CR towards this substance is lower than 1% (Table 3). For the metabolite iminostilbene, also a very low CR was observed. CRs against other pharmaceuticals like antihistamines (CET and loratadine), an antidepressant (opipramol) and another anticonvulsant (Ox-CBZ) are lower than 1%, too, and therefore negligible.
The CR against 10-OH-CBZ (3.0%) is negligible, especially when taking into consideration the excretion of this compound of less than 0.1%.8 The CRs of 3- and 2-OH-CBZ are higher with 37 and 50%, respectively. Associated with the presence in human excretion of 5.1 and 4.3%, respectively, only slight overestimations are expected for the determination of CBZ in water samples.8
CBZ-triglycine was used to synthesize the immunogen and tracers for ELISA and FPIA. Therefore it was expected to show a high CR (94%). There is no natural occurrence of this substance. Although a high CR was found against DiH-CBZ (110%), no overestimations are expected due to this compound, because it occurs neither in human metabolism nor has it been found in any kind of water sample.15 The presence of Ep-CBZ may lead to slight overestimations due to its high CR (120%). The excretion of this substance is approximately one tenth of the CBZ excretion (1.4% compared to 13.8%).8
Compared to a commercially available monoclonal anti-CBZ antibody that has been used for the determination of CBZ in environmental samples, CRs against CBZ-related substances are in a comparable range.19 CRs of the antibody presented in this study are especially advantageous regarding other pharmaceuticals. Loratadine and opipramol were recognized with approximately 2% compared to CBZ by the commercially available antibody (at pH 9.5),19 whereas the new antibody showed CRs lower than 0.1% against these two pharmaceuticals. Especially beneficial is the low recognition of CET by the new antibody. This antihistaminic drug with a strongly deviating structure to CBZ was highly recognized by the commercially available antibody with CRs up to 400%.19 Using the new antibody, this pharmaceutical was not recognized by the antibody under the chosen assay conditions. Other structurally to CET related pharmaceuticals like norchlorcyclizine, hydroxyzine and cloperastine showed CR of 114, 41 and 13%, respectively applying the commercially available antibody.19 Due to the fact that CET was not or only slightly recognized by the new antibody, it can be concluded that also these pharmaceuticals will not or only to a negligible degree be recognized.
In summary, it can be said that the antibody only showed CRs towards CBZ-related substances. The CRs towards substances with relevant concentrations in human metabolism and consequently in water samples are mostly very low and therefore the possibility for an accurate determination of CBZ in these samples is given when this antibody is used.
Conclusion
The applicability of improved strategies for the production and characterization of new monoclonal antibodies could be successfully proven. It could be shown that examinations of antibodies in feces and in conventional blood samples are in good agreement. Feces screening is an animal-friendly alternative to blood sampling and allows time-resolved monitoring of the immune response. The properties of antibodies from cell culture supernatants and purified antibodies were determined using FPIA and ELISA. A good agreement between these methods was found. Therefore the application of FPIA should be considered for a more time-efficient cell culture supernatant screening. Additionally, it could be shown that the reaction time, binding properties and also fluorescence quenching vary significantly between different antibodies. All these antibody properties can easily and rapidly be determined using FPIA for characterization of purified antibodies, but also at the stage of cell culture supernatant screening. The application of this homogeneous assay would therefore simplify the selection of antibodies with desired properties.A new monoclonal anti-carbamazepine (CBZ) antibody was produced and characterized for the application in FPIA and ELISA. With the finally selected antibody (clone 1), sensitive immunoassays could be established. Using FPIA in cuvettes, CBZ concentrations in the range of 1.4–79 μg L−1 can be determined after an incubation time of 5 min and with a test midpoint of 8.9 μg L−1. This assay allows a fast and automated CBZ determination of single samples. FPIA on MTPs allows a simultaneous determination of 24 samples in a total assay time of 20 min within the concentration range of 0.66–110 μg L−1 and a test midpoint of 6.2 μg L−1. With the ELISA format, a more sensitive, but more time-consuming assay could be developed; here, a measurement range of 0.05–36 μg L−1 and a test midpoint of 0.32 μg L−1 could be reached. The CR of the purified antibody was determined by ELISA and FPIA. Most of the determined values are in good agreement, but for some cross-reactants, the different pH values used for the assays influence the CR. For DiH-CBZ, the kind of immunoassay (heterogeneous and homogeneous) seems to influence the binding affinity of the antibody. The antibody showed a high time dependency of CRs and the assay performance including characteristic parameters. Assay performance at the non-equilibrium state improved the sensitivity and selectivity. In general, the determined CRs indicate a good specificity of the antibody and enable for future application in medical and environmental analyses.
The antibody can be requested from the corresponding author. It was assigned the ordering code BAM-mab 01 (CBZ).
Acknowledgements
We express our gratitude to K. Hoffmann for the help for ELISA measurements and S. Flemig and S. Ewald for the MALDI-TOF-MS measurements (all BAM). We also thank Jörg Schenk at hybrotec for assistance with the immunization. This work was supported by a grant from the Federal Ministry of Economic Affairs and Energy (BMWi; program MNPQ, project no. 22/11).References
- D. M. Jameson and J. A. Ross, Chem. Rev., 2010, 110, 2685–2708 CrossRef CAS PubMed.
- D. S. Smith and S. A. Eremin, Anal. Bioanal. Chem., 2008, 391, 1499–1507 CrossRef CAS PubMed.
- J. J. Carvalho, M. A. Walter, Y. Baermann-Stapel, M. G. Weller, U. Panne, J. A. Schenk and R. J. Schneider, In Vivo, 2012, 26, 63–70 CAS.
- G. Köhler and C. Milstein, Nature, 1975, 256, 495–497 CrossRef.
- A. Y. Kolosova, J. H. Park, S. A. Eremin, S. J. Kang and D. H. Chung, J. Agric. Food Chem., 2003, 51, 1107–1114 CrossRef CAS PubMed.
- P. Önnerfjord, S. Eremin, J. Emneus and G. Marko-Varga, J. Immunol. Methods, 1998, 213, 31–39 CrossRef.
- S. K. Bedada and P. Nearati, Phytother. Res., 2015, 29, 701–706 CrossRef CAS PubMed.
- A. Bahlmann, W. Brack, R. J. Schneider and M. Krauss, Water Res., 2014, 57, 104–114 CrossRef CAS PubMed.
- M. Kahle, I. J. Buerge, M. D. Müller and T. Poiger, Environ. Toxicol. Chem., 2009, 28, 2528–2536 CAS.
- E. R. V. Dickenson, S. A. Snyder, D. L. Sedlak and J. E. Drewes, Water Res., 2011, 45, 1199–1212 CrossRef CAS PubMed.
- A. Almeida, V. Calisto, V. I. Esteves, R. J. Schneider, A. M. V. M. Soares, E. Figueira and R. Freitas, Aquat. Toxicol., 2014, 156, 74–87 CrossRef CAS PubMed.
- B. Ferrari, N. Paxeus, R. Lo Giudice, A. Pollio and J. Garric, Ecotoxicol. Environ. Saf., 2003, 55, 359–370 CrossRef CAS PubMed.
- T. A. Ternes, J. Stüber, N. Herrmann, D. McDowell, A. Ried, M. Kampmann and B. Teiser, Water Res., 2003, 37, 1976–1982 CrossRef CAS PubMed.
- R. I. L. Eggen, J. Hollender, A. Joss, M. Schärer and C. Stamm, Environ. Sci. Technol., 2014, 48, 7683–7689 CrossRef CAS PubMed.
- A. Bahlmann, M. G. Weller, U. Panne and R. J. Schneider, Anal. Bioanal. Chem., 2009, 395, 1809–1820 CrossRef CAS PubMed.
- J. Grandke, L. Oberleitner, U. Resch-Genger, L.-A. Garbe and R. J. Schneider, Anal. Methods, 2013, 5, 3754–3760 RSC.
- W. Y. Lin, M. L. Pan, H. Y. Wang, Y. O. Su and P. W. Huang, Med. Chem. Res., 2012, 21, 4389–4394 CrossRef CAS.
- L. Oberleitner, S. A. Eremin, A. Lehmann, L.-A. Garbe and R. J. Schneider, Anal. Methods, 2015, 7, 5854–5861 RSC.
- A. Bahlmann, J. Falkenhagen, M. G. Weller, U. Panne and R. J. Schneider, Analyst, 2011, 136, 1357–1364 RSC.
- A. Dasgupta, G. Tso, M. Johnson and L. Chow, Ther. Drug Monit., 2010, 32, 112–115 CrossRef CAS PubMed.
- J. Grandke, L. Oberleitner, U. Resch-Genger, L.-A. Garbe and R. J. Schneider, Anal. Bioanal. Chem., 2013, 405, 1601–1611 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- A. Frey, B. Meckelein, D. Externest and M. A. Schmidt, J. Immunol. Methods, 2000, 233, 47–56 CrossRef CAS PubMed.
- R. Ekins, Ligand Q., 1981, 4, 33–44 Search PubMed.
- L. Oberleitner, J. Grandke, F. Mallwitz, U. Resch-Genger, L.-A. Garbe and R. J. Schneider, J. Agric. Food Chem., 2014, 62, 2337–2343 CrossRef CAS PubMed.
- C. Tan, N. Gajovic-Eichelmann, R. Polzius, N. Hildebrandt and F. F. Bier, Anal. Bioanal. Chem., 2010, 398, 2133–2140 CrossRef CAS PubMed.
- U. Eisold, F. Sellrie, J. A. Schenk, C. Lenz, W. F. M. Stöcklein and M. U. Kumke, Anal. Bioanal. Chem., 2015, 407, 3313–3323 CrossRef CAS PubMed.
- C. Schneider, H. F. Schöler and R. J. Schneider, Anal. Chim. Acta, 2005, 551, 92–97 CrossRef CAS.
- M. G. Weller, L. Weil and R. Niessner, Mikrochim. Acta, 1992, 108, 29–40 CrossRef CAS.
- A. Y. Kolosova, J.-H. Park, S. A. Eremin, S.-J. Park, S.-J. Kang, W.-B. Shim, H.-S. Lee, Y.-T. Lee and D.-H. Chung, Anal. Chim. Acta, 2004, 511, 323–331 CrossRef CAS.
- Z. L. Xu, Q. Wang, H. T. Lei, S. A. Eremin, Y. D. Shen, H. Wang, R. C. Beier, J. Y. Yang, K. A. Maksimova and Y. M. Sun, Anal. Chim. Acta, 2011, 708, 123–129 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2. See DOI: 10.1039/c6ay01968d |
| This journal is © The Royal Society of Chemistry 2016 |