Open Access Article
Open Access ArticleRapid detection of adulterated drugs in herbal dietary supplements by wooden-tip electrospray ionization mass spectrometry†
Bin
Hu
ab,
Yingyan
Huang
ab,
Guo
Yin
c,
Gaofei
Zhang
c,
Liyang
Zhang
c,
Tiejie
Wang
*c and
Zhong-Ping
Yao
*abd
aState Key Laboratory for Chirosciences, Food Safety and Technology Research Centre and Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong Special Administrative Region, China. E-mail: zhongping.yao@polyu.edu.hk; Fax: +852 2364 9932; Tel: +852 3400 8792
bState Key Laboratory of Chinese Medicine and Molecular Pharmacology (Incubation) and Shenzhen Key Laboratory of Food Biological Safety Control, Shenzhen Research Institute of The Hong Kong Polytechnic University, Shenzhen, 518057, China
cShenzhen Institute for Drug Control, Shenzhen, 518057, China. E-mail: wangtj88@163.com; Fax: +86 755 26031729; Tel: +86 755 26031728
dKey Laboratory of Natural Resources of Changbai Mountain and Functional Molecules (Yanbian University), Ministry of Education, Yanji, 133002, China
First published on 5th August 2016
Abstract
Adulteration of herbal dietary supplements with unlabelled drugs is illegal and can lead to serious health problems in consumers. In this study, wooden-tip electrospray ionization mass spectrometry (WT-ESI-MS) was developed for rapid detection of adulterated drugs in three categories of herbal dietary supplements, namely tranquilizer, aphrodisiac and weight-loss products. Samples were directly taken by using pre-wetted wooden tips and analyzed by WT-ESI-MS, with the drug identities confirmed by MS/MS spectra. Furthermore, a high-throughput WT-ESI-MS method was developed to allow automated WT-ESI-MS and MS/MS analysis of herbal dietary supplement samples with an analysis speed of ∼15 seconds per sample. The limits of detection of typical drugs were found to be at the ng g−1 level using the WT-ESI-MS method. In this study, 33 commonly adulterated drugs were investigated and a spectral database containing the ESI-MS and ESI-MS/MS spectra of these drug standards was established. A total of 144 herbal dietary supplements were analyzed and the results were compared with those obtained by using high performance liquid chromatography. These results demonstrated a simple and comprehensive approach for rapid detection of adulterated drugs in herbal dietary supplements.
Introduction
Herbal dietary supplements are becoming more and more popular around the world. Botanicals and botanical preparations are widely used to maintain or improve health, with public beliefs that natural herbal dietary supplements are safe and have no side effects.1 It has been reported that the sales of herbal dietary supplements in the USA reached an estimated total of more than 6.4 billion USD in 2014, marking the 11th consecutive year of growth.2 However, illegal adulteration of herbal dietary supplements with unlabelled drugs has been frequently reported.3–7 The adulterations typically involve adding unlabelled drugs into corresponding herbal supplements, e.g., tranquilizer, aphrodisiac and weight-loss products, aiming to make them show faster and more significant pharmaceutical effects and increase the sales of the products. Sibutramine and sildenafil are among the most frequently used drugs for this purpose.5,8 Such adulterations have become public health concerns, considering both the booming consumption of herbal dietary supplements and the fact that the consumption of unlabelled drugs could cause serious adverse health effects or even death.9–13 Therefore, the development of novel techniques for rapid, sensitive, effective detection of adulterated drugs in herbal dietary supplements is of paramount importance.Various analytical techniques, including liquid chromatography (LC), gas chromatography (GC), thin-layer chromatography, flow injection and capillary electrophoresis, have been used for the detection of adulterated drugs in herbal dietary supplements.7,14 To obtain more reliable results, mass spectrometry (MS), which is usually coupled with GC and LC, was employed due to its desirable sensitivity and specificity.15,16 Because of the complicated matrices in herbal dietary supplements, extensive sample pre-treatment such as extraction, pre-concentration, separation, and derivatization, which could be very time-consuming, is usually required prior to MS detection. Recently, ambient MS techniques including desorption electrospray ionization (DESI),17 direct analysis in real time (DART),18 desorption atmospheric pressure chemical ionization (DAPCI),19 desorption corona beam ionization (DCBI),20 extractive electrospray ionization (EESI),21 low-temperature plasma (LTP),22 and paper spray ionization (PSI)23 have been developed for analysis of raw samples with minimal or no sample pre-treatment and no chromatographic separation. Two such techniques, DART and DCBI, which generated plasma upon corona discharge for desorption/ionization of analytes on the sample surfaces, have been employed for rapid screening of adulterated drugs in herbal dietary supplements after some simple sample pre-treatments.18,20
In the past few years, we have developed several electrospray ionization (ESI)-based ambient MS techniques to facilitate analysis of raw samples. Wooden-tip ESI-MS (WT-ESI-MS)24 is such a technique that could be used for direct analysis of raw samples.25–31 This technique makes use of readily available, economical and disposable wooden toothpicks, which can be directly compatible with commercially available nanoESI ion sources, for sampling and ionization. The slim and hard wooden tips are very convenient for sampling, and the techniques could be used for analysis of samples of various forms.32–37
In this study, WT-ESI-MS was further developed for rapid detection of adulterated drugs in three common categories of herbal dietary supplements, i.e., tranquilizer, aphrodisiac and weight-loss products. Thirty-three commonly adulterated drugs were analyzed with their standards, and a database containing their ESI-MS and ESI-MS/MS spectra was established. The WT-ESI-MS technique was applied to analyse 144 herbal dietary supplements, with 18 drugs found and the results compared with those obtained by LC-based analysis. High-throughput WT-ESI-MS was also developed to couple with automated MS/MS for rapid screening. The results present a simple and comprehensive approach for rapid detection of adulterated drugs in herbal dietary supplements.
Experimental
Chemicals and materials
All standard drugs were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Methanol, formic acid (FA) and acetonitrile (ACN) were purchased from Fisher Scientific (Waltham, MA, USA). Ammonium acetate, acetic acid, triethylamine, phosphoric acid and phosphate buffer were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). Water used in this study was Milli-Q water (Millipore, Billerica, MA, USA). Herbal dietary supplements (pills, tablets, capsules or soft-gel capsules) were collected from markets by Shenzhen Institute for Drug Control (Shenzhen, China) and were prepared in powder forms except for the soft-gel capsules. Wooden tips (toothpicks, OD: 2.0 mm) were purchased from ParknShop (Hong Kong) and were cut into tips each with a length of 2 cm and a tip size of 0.2 mm before use. Insulative soft plastic bricks (H × W × L: 1.15 × 2.15 × 6.25 cm) were purchased from Faber-Castell Inc. (Kuala Lumpur, Malaysia), stainless steel needles (L: 24 mm, OD: 0.45 mm) from Suzhou Acupuncture and Moxibustion Appliance Co. Ltd. (Suzhou, China), a motorized positioning system from KD Scientific Inc. (Holliston, MA, USA), and a supporting platform device from Beijing Zolix Instruments Co., Ltd (Beijing, China).Conventional ESI-MS
Thirty-three standard drugs were prepared in methanol solutions (1.0 μg mL−1) and analysed by conventional ESI-MS (Q-TOF2, Waters) to obtain their ESI-MS and ESI-MS/MS spectra. The ionization voltage and cone voltage were set at 3.0 kV and 30 V, respectively. Collision energy was set at 15–45 V in the MS/MS experiments. The flow rate of the sample was 5.0 μL min−1. Nitrogen was used as the sheath gas, cone gas and desolvation gas with a flow rate of 15 L h−1, 75 L h−1 and 250 L h−1, respectively.WT-ESI-MS
Wooden-tip ESI-MS analysis was performed similarly to our previous work.24,32 Briefly, a wooden tip was pre-wetted in Milli-Q water for the sampling of powder samples, or directly used to penetrate the soft-gel capsule for the sampling of the liquid sample in the soft-gel capsule. The wooden tip with the sample loaded was mounted onto the capillary holder of the nanoESI ion source in a Waters Q-TOF2 mass spectrometer. Subsequently, 5.0 μL optional solvent was pipetted onto the tip surface, and a high voltage of 3.5 kV was supplied to the tip via the nanoESI ion source to generate spray ionization. The conditions of MS/MS experiments were set the same as those in conventional ESI-MS/MS.High-throughput WT-ESI-MS and automated MS/MS
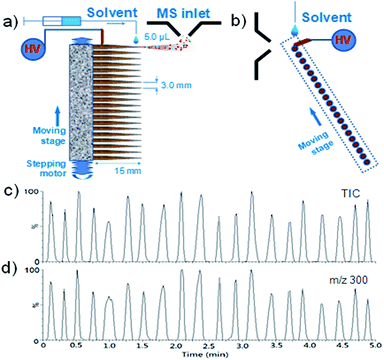
A device that allows automated analysis of 20 sample-loaded wooden tips each time was designed and fixed on a supporting platform in the front of the MS inlet for high-throughput analysis in this study (see Fig. 1). In this device, a batch of 20 wooden tips were put in the soft plastic bricks with a distance of 3.0 mm between two adjacent tip ends. Each wooden tip was 2.0 cm in length, with 0.5 cm inserted into the soft plastic bricks for fixing the tip and the other 1.5 cm for loading the sample and solvent (Fig. 1a). For sample analysis, the soft plastic brick with 20 sample-loaded wooden tips was mounted on the motorized positioning system and moved at a speed of 0.2 mm s−1. When each tip moved to the MS inlet, it was connected at a high voltage of 3.5 kV via an elastic stainless steel needle, and at the same time, a droplet of 5.0 μL solvent, which was supplied by using a syringe with a syringe pump, was loaded onto the wooden tip, leading to spray ionization and generation of a spectrum for the sample on the tip (Fig. 1b). The soft plastic brick could be reused for holding disposable wooden tips, and another brick with 20 sample-loaded tips could be placed on the system for automated sample analysis after the previous one. Automated MS/MS was performed for screening of various drugs simultaneously using Waters Masslynx 4.1. The scan time and inter-scan delay were set at 0.5 s and 0.1 s, respectively. Collision energy (CE) was set the same as that in the MS/MS experiments for the standard drugs.High-performance liquid chromatography (HPLC) analysis
All standard drugs were prepared in 0.1 mg mL−1 in methanol for the HPLC analysis with a diode-array detector (DAD) as the detector. All herbal dietary supplement samples were prepared in the following steps: (1) each sample was individually weighed and then dissolved in 25.0 mL methanol for ultrasonic processing (120 W, Shenzhen Boda Ultrasonic Cleaning Equipment Co., Ltd, Shenzhen, China) for 15 min; (2) methanol extracts of the samples were filtered twice by using a filter membrane (0.45 μm, Tianjin Jinteng Experiment Equipment Co., Ltd, Tianjin, China). Methanol extracts of the samples and the standard solutions were analyzed by HPLC-DAD (Waters Alliance e2695-2998) under the following conditions: a Poroshell 120 EC-C18 column (2.7 μm, 4.6 mm × 50 mm, Waters), a GL. Insersil ODS-3 column (5 μm, 250 mm × 4.6 mm, Waters) and a DIKMA Diamonsil-C18 column (ODS, 5 μm, 4.6 mm × 250 mm, Dikma Technologies, Beijing, China) were used for analysis of the tranquilizer, aphrodisiac and weight-loss standards and samples, respectively. The temperatures of the columns were set at 35 °C. The detection wavelength of the DAD was 230 nm for both tranquilizer and aphrodisiac samples, and 225 nm or 265 nm for the weight-loss samples. For the tranquilizer samples, ACN was used as mobile phase A and phosphate buffer (0.01 mol L−1, pH: 2.5) as mobile phase B, with the following gradient setting: 0–20 min, A: 5 → 25%, B: 95 → 75%; 20–30 min, A: 25%, B: 75%; 30–35 min, A: 25 → 40%, B: 75 → 60%; 35–36 min, A: 40 → 5%, B: 60 → 95%. For the aphrodisiac samples, ACN was used as mobile phase A and triethylamine-phosphate acid solution (0.05 mol L−1, pH: 3.0) as mobile phase B with the following gradient setting: 0–15 min, A: 27%, B: 73%; 15–30 min, A: 27 → 40%, B: 73 → 60%; 30–40 min, A: 40 → 80%, B: 60 → 20%; 40–50 min, A: 80%, B: 20%; 50–53 min, A: 80 → 27%, B: 20 → 73%; 53–60 min, A: 27%, B: 73%). For the weight-loss samples, acetic acid (0.1%)–ammonium acetate (0.02 mol L−1) buffering solution was used as mobile phase A and methanol as mobile phase B, with the following gradient setting: 0–10 min, A: 75 → 65%, B: 25 → 35%; 10–20 min, A: 65 → 40%, B: 35 → 60%; 20–28 min, A: 40 → 20%, B: 60 → 80%; 28–37 min, A: 20%, B: 80%; 38–48 min, A: 10%, B: 90%; 48–50 min, A: 10 → 75%, B: 90 → 25%; 50–55 min, A: 75%, B: 25%. The flow rates of the mobile phases were set at 0.8 mL min−1.Results and discussion
ESI-MS and MS/MS analysis of 33 standard drugs
In this study, 33 drugs that are commonly adulterated in the three categories of herbal dietary supplements, including 17 tranquilizer drugs, 11 aphrodisiac drugs, and 5 weight-loss drugs, were analyzed with their standards by conventional ESI-MS and MS/MS, in order to set up a spectral database for the structural confirmation of drug analytes detected from the samples. Protonated molecules [M + H]+ were observed for all these standard compounds with positive ion ESI-MS, and these protonated molecules were selected for collision-induced dissociation to generate characteristic MS/MS spectra for each drug. Some drugs, i.e., sulfoaildenafil and hydroxyhomosildenafil, produced very similar protonated molecules (e.g., m/z 505) in their ESI-MS spectra, but their MS/MS spectra were very different and allowed them to be distinguished and determined (Fig. S2g and h†). The monoisotopic masses, molecular structures, ESI-MS and MS/MS spectra of these 33 standard drugs are collectively shown in Fig. S1–S3,† and their MS/MS data are also summarized in Tables S4–S6.†Analysis of herbal dietary supplement samples by WT-ESI-MS
WT-ESI-MS was applied to analyze the three categories of herbal dietary supplements collected from markets. A typical spectrum is shown in Fig. 2a for one tranquilizer product (sample no. A20), obtained with methanol as the extraction and ionization solvent. The peaks at m/z 219, 365, 381 and 399 corresponded to the salt adducts of sugars [glucose + K]+, [sucrose + Na]+, [sucrose + K]+ and [2glucose + K]+, respectively, which were commonly observed for herbal samples. The peak at m/z 300 predominated in the spectrum and was selected for the MS/MS experiment. Product ions at m/z 283, 266, 255 and 241 were observed in the MS/MS spectrum (Fig. 2b), in good agreement with the MS/MS data of the chlordiazepoxide standard (Fig. S1g†) and the literature.38 Chlordiazepoxide was thus determined in sample no. A20.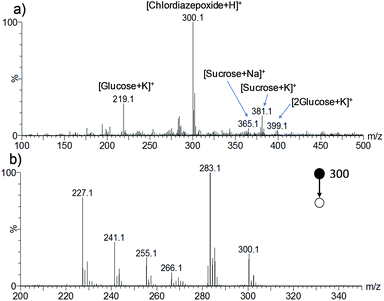 | ||
| Fig. 2 Spectra obtained by analysis of tranquilizer sample no. A20 with WT-ESI-MS: (a) the full mass spectrum; (b) the MS/MS spectrum of the peak at m/z 300 in (a). | ||
Selection of solvents for sample extraction and ionization
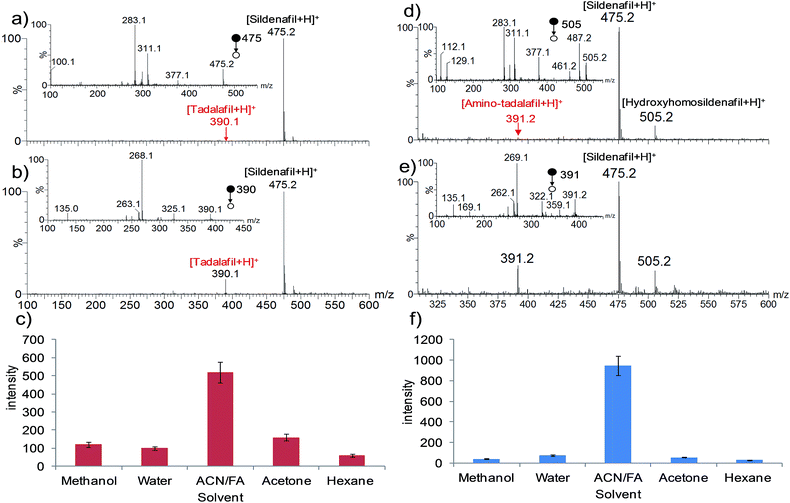
Methanol was used as the solvent for ESI-MS analysis of the standard drugs (1.0 μg mL−1) in this study and was also employed as the extraction and ionization solvent for WT-ESI-MS analysis of the herbal dietary supplement samples. However, it was found that poor responses were observed for the WT-ESI-MS detection of some drug analytes, e.g., tadalafil, from the samples, presumably due to the limited solubility of the drugs in methanol39 and thus low efficiencies of extraction from the raw samples. For example, an aphrodisiac sample, no. Z19, was detected to be adulterated with sildenafil and tadalafil, two drugs for clinically treating erectile dysfunction,5 by HPLC-DAD analysis. This sample was analyzed by WT-ESI-MS using methanol as the extraction and ionization solvent, and the obtained spectrum is shown in Fig. 3a. The base peak at m/z 475 in the full spectrum was identified as sildenafil based on the mass and the MS/MS results (see the inset in Fig. 3a for the MS/MS spectrum). However, tadalafil, with [M + H]+ at m/z 390, was detected with an abundance below 1% in the spectrum. Different solvents were thus tested to optimize the detection of tadalafil in the sample. As shown in Fig. 3c, the best response of tadalafil was obtained with acetonitrile containing 0.1% formic acid (ACN/FA) as the solvent. The use of this solvent allowed the detection of both sildenafil and tadalafil in the sample by WT-ESI-MS, with tadalafil confirmed by the MS/MS spectrum (Fig. 3b). Another case is shown in Fig. 3d–f. Two adulterated drugs, i.e., sildenafil and hydroxyhomosildenafil, were clearly detected in aphrodisiac sample no. Z25 by WT-ESI-MS with methanol as the extraction and ionization solvent (Fig. 3d). However, amino-tadalafil, which was detected in the sample by HPLC-DAD analysis, showed a very low abundance in the spectrum. The optimized solvent (see Fig. 3f for the optimization result) allowed much enhanced detection of aminotadalafil (m/z 391) along with the two other drugs. The solvent acetonitrile containing 0.1% formic acid was used for WT-ESI-MS analysis of other samples as well and was found to improve the detection of tadalafil-containing samples, including samples no. Z4, Z7, Z9, Z12, Z15, Z16, Z17, Z19 and Z20.Comparison of the results with those obtained by HPLC-DAD analysis
In this study, 144 herbal dietary supplement samples, including 43 tranquilizer samples, 57 aphrodisiac samples and 44 weight-loss samples, were investigated using WT-ESI-MS. The detection results are summarized in Tables S1–S3,† with comparison with the results obtained by HPLC-DAD analysis. It could be seen that all drugs detected by the HPLC-DAD method could also be detected by the WT-ESI-MS method, while the WT-ESI-MS method allowed the detection of additional drugs in some samples, e.g., chlorpheniramine in sample A25, melatonin in sample A38, sildenafil and hydroxyhomosildenafil in sample Z25, sibutramine in J19 and J34, and sibutramine in sample J42 (labelled in italics and red in Tables S1–S3†). The better detection of the drugs in the supplement samples by the WT-ESI-MS method was probably because MS was more sensitive and universal than DAD for the detection of the analytes and the WT-ESI-MS method allowed direct analysis of the raw samples, avoiding potential analyte losses during the sample processing. These results demonstrated that WT-ESI-MS could be a reliable method for the detection of adulterated drugs in herbal dietary supplements. Compared to the HPLC-DAD method that required extensive sample pre-treatment and chromatographic separation and thus typically took several hours for analysis of one sample, the WT-ESI-MS method was much simpler, used much less solvent and consumables, and could complete the analysis of one sample within minutes.As shown in Tables S1–S3,† various adulterated drugs were detected in the collected herbal dietary supplement samples, including melatonin, doxepin, diazepam, chlorpheniramine, zopiclone, nitrazepam, zaleplon, alprazolam, clonazepam and chlordiazepoxide in the tranquilizer samples, mainly sildenafil and also vardenafil, tadalafil, amino tadalafil and hydroxyhomosildenafil in the aphrodisiac samples, and sibutramine and phenolphthalein in the weight-loss samples. It was noted that sildenafil, an aphrodisiac synthetic drug, was detected in a weight-loss dietary supplement (sample no. J34) by both the HPLC-DAD and WT-ESI-MS methods, indicating the complexity of adulteration and the need to develop comprehensive and non-targeted detection methods.
Limit of detection of the method
The limit of detection (LOD) of the method was determined by measuring two typical drugs for each category of herbal dietary supplements, i.e., diazepam and zopiclone for the tranquilizer samples, sildenafil and amino-tadalafil for the aphrodisiac samples, and sibutramine and phenolphthalein for the weight-loss samples, in this study. For the measurement, an aliquot of 10.0 μL standard solution of each drug was mixed with 10 mg starch and dried in air for analysis as a powder sample. Starch is commonly used for manufacturing and filling capsules and tablets in the pharmaceutical industry40,41 and was used as the matrix substrate in this study. Blank samples, i.e., starch samples without drugs spiked, were analyzed as well for comparison and the signal-to-noise (S/N) determination. Each drug was detected with a concentration of 0.1 μg g−1 in the substrate, and their S/N values as determined based on the MS/MS signals of their major fragments are shown in Table 1. It could be found that the S/N values of all six drugs were better than 3, revealing that the LOD of the detection method was better than 0.1 μg g−1 for the drugs and the method allowed the detection of adulteration of trace drugs in herbal dietary supplements.| Category of dietary supplement | Druga | MS/MS detection | Drug concentration (μg g−1) | S/N (n = 3) |
|---|---|---|---|---|
| a Methanol was used as the extraction and ionization solvent, except acetonitrile containing 0.1% formic acid for amino-tadalafil. | ||||
| Tranquilizer | Diazepam | 285 > 193 | 0.1 | 6.2 ± 0.8 |
| Zopiclone | 389 > 245 | 0.1 | 9.4 ± 1.4 | |
| Aphrodisiac | Sildenafil | 475 > 283 | 0.1 | 25.2 ± 4.0 |
| Amino-tadalafil | 391 > 269 | 0.1 | 7.7 ± 1.0 | |
| Weight-loss | Sibutramine | 280 > 139 | 0.1 | 3.6 ± 0.3 |
| Phenolphthalein | 319 > 225 | 0.1 | 18.2 ± 2.0 | |
High-throughput analysis of herbal dietary supplements
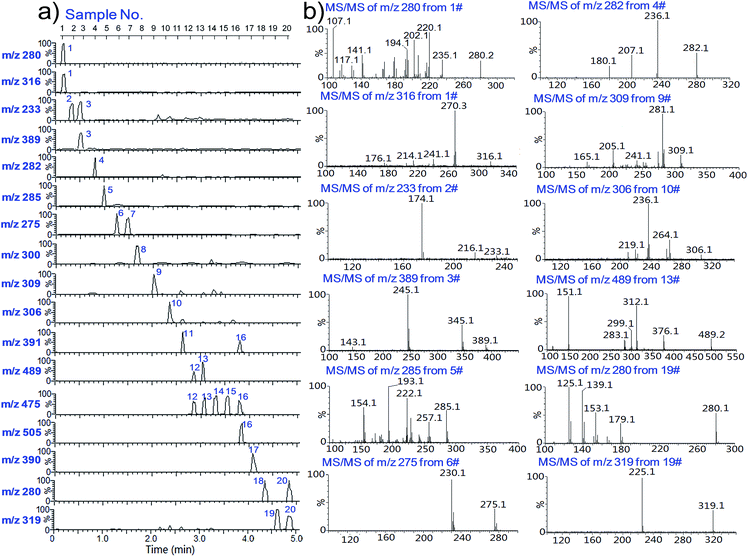
To further improve the analytical efficiency of the method, a high-throughput WT-ESI-MS device was developed in this study. Different from our previous high-throughput device,42 which is applicable for mass spectrometers that apply a high voltage to the MS inlet to induce electrospray ionization (e.g., Agilent, Bruker equipment),42,43 the present high-throughput device was designed for mass spectrometers that apply the high voltage to the sample part to induce the ionization (e.g., Waters, AB Sciex and Thermo equipment). As shown in Fig. 1a and b, this device allowed automated analysis of a batch of 20 wooden-tip samples each time, and a new batch of samples could be placed for another automated analysis after that. The performance of this device for analysis of herbal dietary supplements was investigated using an herbal tranquilizer sample (sample no. A20) as an example. A batch of 20 wooden tips loaded with sample A20 were analyzed by the technique, and the total ion current (TIC) and selected ion current (SIC) chronograms of m/z 300, which corresponded to [M + H]+ of chlordiazepoxide, recorded for the 20 measurements are shown in Fig. 1c and d. The results demonstrated that the adulterated drug in each sample could be effectively detected with an analysis speed of ∼15 seconds per sample. The signal response of each tip was related to the sample amount loaded onto the tip. The relative standard deviation (RSD) of the signals for these 20 measurements was found to be 19.6%, which was comparable to that of DBCI-MS (RSD: 8–25%) for direct analysis of powder herbal dietary supplements.20 The introduction of an internal standard into the sample could compensate for the signal fluctuations and improve the technique for quantification, if necessary.The high-throughput WT-ESI-MS device was further coupled with multi-channel and automated MS/MS to allow simultaneous screening of different adulterated drugs in the samples and confirmatory determination of the detected drugs. In this study, 20 different samples were analyzed for simultaneous screening of 17 drugs as a demonstration of the technique. Fig. 4a shows the 17 chronograms obtained by simultaneous screening of 17 drugs for the 20 samples. The detected drug ions were automatically selected for MS/MS analysis by the software, with some of the spectra shown in Fig. 4b. For example, the 1st and 2nd chronograms indicated the detection of drug ions m/z 280 and m/z 316 in sample #1, and the subsequent automated MS/MS of these two ions and the comparison of the acquired spectra (Fig. 4b) with those in the database (Fig. S1–S3†) confirmed the presence of doxepin and clonazepam in the sample. These results demonstrated that the automated wooden-tip ESI-MS and MS/MS design could provide a high-throughput and effective approach for screening of various adulterated drugs in herbal dietary supplements.
Conclusions
The determination of adulterated drugs in herbal dietary supplements requires rapid and reliable detection techniques, typically without the need for quantitation since adulteration of unlabelled drugs in herbal dietary supplements is prohibited. In this study, an ESI-MS and MS/MS spectral database of 33 commonly adulterated drugs was established, and a WT-ESI-MS-based method was developed for rapid detection of drugs in herbal dietary supplements. The technique made use of economical and disposable wooden toothpicks, required minimum sample preparation and no chromatographic separation, and allowed the detection of trace drugs in herbal dietary supplements and the confirmation of the detected drugs with MS/MS spectra. Analysis of 144 herbal dietary supplement samples demonstrated the effectiveness of the method. A high-throughput WT-ESI-MS coupled with automated MS/MS was further developed for fast screening of adulterated drugs in herbal dietary supplements. These results demonstrated a simple, economical, and effective strategy for the detection of adulterated drugs in herbal dietary supplements, which could be extended for real applications.Acknowledgements
This work was supported by the Hong Kong, Macau and Taiwan Science & Technology Cooperation Program of China (Grant No. 2014DFH30160), Natural Science Foundation of China (Grants No. 81373369 and No. 21405127), and Hong Kong Research Grants Council (Grant No. 5029/13P).References
- S. R. B. M. Eussen, H. Verhagen, O. H. Klungel, J. Garssen, H. van Loveren, H. J. van Kranen and C. J. M. Rompelberg, Eur. J. Pharmacol., 2011, 668, S2–S9 CrossRef CAS PubMed.
- T. Smith, M. Lynch, J. Johnson, K. Kawa, H. Bauman and M. Blumenthal, HerbalGram, 2015, 107, 52–59 Search PubMed.
- E. Ernst, J. Intern. Med., 2002, 252, 107–113 CrossRef CAS PubMed.
- E. Ernst, Trends Pharmacol. Sci., 2002, 23, 136–139 CrossRef CAS PubMed.
- T. Rocha, J. S. Amaral and M. B. P. P. Oliveira, Compr. Rev. Food Sci. Food Saf., 2016, 15, 43–62 CrossRef.
- B. Guo, M. L. Wang, Y. Y. Liu, J. Zhou, H. Dai, Z. Q. Huang, L. L. Shen, Q. S. Zhang and B. Chen, J. Agric. Food Chem., 2015, 63, 6954–6967 CrossRef CAS PubMed.
- J. Calahan, D. Howard, A. J. Almalki, M. P. Gupta and A. I. Calderon, Planta Med., 2016, 82, 505–515 CrossRef CAS PubMed.
- Y. Chen, L. Zhao, F. Lu, Y. Yu, Y. Chai and Y. Wu, Food Addit. Contam., Part A, 2009, 26, 595–603 CrossRef CAS PubMed.
- P. A. Chyka, Am. J. Med., 2000, 109, 122–130 CrossRef CAS PubMed.
- M. W. Fischman and N. K. Mello, Drug Alcohol Depend., 1991, 28, 1–2 CrossRef.
- H. Dewit and R. R. Griffiths, Drug Alcohol Depend., 1991, 28, 83–111 CrossRef CAS.
- A. A. Savaliya, R. P. Shah, B. Prasad and S. Singh, J. Pharm. Biomed. Anal., 2010, 52, 406–409 CrossRef CAS PubMed.
- B. J. Venhuis and D. de Kaste, J. Pharm. Biomed. Anal., 2012, 69, 196–208 CrossRef CAS PubMed.
- L. Vaclavik, A. J. Krynitsky and J. I. Rader, Anal. Bioanal. Chem., 2014, 406, 6767–6790 CrossRef CAS PubMed.
- K. Reddersen and T. Heberer, J. Sep. Sci., 2003, 26, 1443–1450 CrossRef CAS.
- K. Mitrowska, A. Posyniak and J. Zmudzki, Anal. Chim. Acta, 2009, 637, 185–192 CrossRef CAS PubMed.
- J. F. Garcia-Reyes, A. U. Jackson, A. Molina-Diaz and R. G. Cooks, Anal. Chem., 2009, 81, 820–829 CrossRef CAS PubMed.
- Z. G. Zhou, J. L. Zhang, W. Zhang, Y. Bai and H. W. Liu, Analyst, 2011, 136, 2613–2618 RSC.
- S. P. Yang, J. H. Ding, J. Zheng, B. Hu, J. Q. Li, H. W. Chen, Z. Q. Zhou and X. L. Qiao, Anal. Chem., 2009, 81, 2426–2436 CrossRef CAS PubMed.
- H. Wang, Y. Wu, Y. Zhao, W. Sun, L. Ding, B. Guo and B. Chen, Food Addit. Contam., Part A, 2012, 29, 1194–1201 CrossRef CAS PubMed.
- B. Hu, X. J. Peng, S. P. Yang, H. W. Gu, H. W. Chen, Y. F. Huan, T. T. Zhang and X. L. Qiao, J. Am. Soc. Mass Spectrom., 2010, 21, 290–293 CrossRef CAS PubMed.
- J. S. Wiley, J. F. Garcia-Reyes, J. D. Harper, N. A. Charipar, Z. Ouyang and R. G. Cooks, Analyst, 2010, 135, 971–979 RSC.
- L. H. Shen, J. Zhang, Q. Yang, N. E. Manicke and Z. Ouyang, Clin. Chim. Acta, 2013, 420, 28–33 CrossRef CAS PubMed.
- B. Hu, P. K. So, H. W. Chen and Z. P. Yao, Anal. Chem., 2011, 83, 8201–8207 CrossRef CAS PubMed.
- B. Hu, P. K. So and Z. P. Yao, Anal. Chim. Acta, 2014, 817, 1–8 CrossRef CAS PubMed.
- B. Hu, G. Z. Xin, P. K. So and Z. P. Yao, J. Chromatogr. A, 2015, 1415, 155–160 CrossRef CAS PubMed.
- B. Hu, Y. H. Lai, P. K. So, H. W. Chen and Z. P. Yao, Analyst, 2012, 137, 3613–3619 RSC.
- H. X. Wang, P. K. So and Z. P. Yao, Anal. Chim. Acta, 2014, 809, 109–116 CrossRef CAS PubMed.
- B. Hu and Z. P. Yao, Anal. Chem., 2016, 88, 5585–5589 CrossRef CAS PubMed.
- P. K. So, B. Hu and Z. P. Yao, Mol. BioSyst., 2013, 9, 915–929 RSC.
- G. Z. Xin, B. Hu, Z. Q. Shi, J. Y. Zheng, L. Wang, W. Q. Chang, P. Li, Z. P. Yao and L. F. Liu, J. Pharm. Biomed. Anal., 2016, 117, 492–498 CrossRef CAS PubMed.
- B. Hu, P. K. So and Z. P. Yao, J. Am. Soc. Mass Spectrom., 2013, 24, 57–65 CrossRef CAS PubMed.
- G. Z. Xin, B. Hu, Z. Q. Shi, Y. C. Lam, T. T. X. Dong, P. Li, Z. P. Yao and K. W. K. Tsim, Anal. Chim. Acta, 2014, 820, 84–91 CrossRef CAS PubMed.
- P. K. So, T. T. Ng, H. X. Wang, B. Hu and Z. P. Yao, Analyst, 2013, 138, 2239–2243 RSC.
- B. C. Yang, F. Wang, W. Deng, Y. Zou, F. Y. Liu, X. D. Wan, X. Yang, H. Liu and O. P. Huang, Anal. Methods, 2015, 7, 5886–5890 RSC.
- B. C. Yang, F. Y. Liu, L. Q. Wang, Y. Zou, F. Wang, W. Deng, X. D. Wan, X. Yang, M. He and O. P. Huang, Anal. Methods, 2015, 7, 6125–6132 RSC.
- B. C. Yang, F. Y. Liu, J. B. Guo, L. Wan, J. Wu, F. Wang, H. Liu and O. P. Huang, Anal. Methods, 2015, 7, 2913–2916 RSC.
- M. L. Nedved, S. HabibiGoudarzi, B. Ganem and J. D. Henion, Anal. Chem., 1996, 68, 4228–4236 CrossRef CAS PubMed.
- S. R. Gratz, C. L. Flurer and K. A. Wolnik, J. Pharm. Biomed. Anal., 2004, 36, 525–533 CrossRef CAS PubMed.
- V. D. Vilivalam, L. Illum and K. Iqbal, Pharm. Sci. Technol. Today, 2000, 3, 64–69 CrossRef CAS PubMed.
- K. Kailasapathy, LWT–Food Sci. Technol., 2006, 39, 1221–1227 CrossRef CAS.
- Y. Y. Yang, J. W. Deng and Z. P. Yao, Anal. Chim. Acta, 2015, 887, 127–137 CrossRef CAS PubMed.
- B. Hu, L. Wang, W. C. Ye and Z. P. Yao, Sci. Rep., 2013, 3, 2104 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3 and Tables S1–S6. See DOI: 10.1039/c6ay01735e |
| This journal is © The Royal Society of Chemistry 2016 |