Open Access Article
Open Access ArticleGeographical differentiation of a monovarietal olive oil using various chemical parameters and mid-infrared spectroscopy
Oguz
Uncu
and
Banu
Ozen
*
Izmir Institute of Technology, Department of Food Engineering, Urla-Izmir, Turkey. E-mail: banuozen@iyte.edu.tr; Fax: +90 232 750 6196
First published on 24th May 2016
Abstract
Increased demand for monovarietal olive oils from local olive varieties with unique characteristics as well as regulations such as ‘Protected Designation of Origin’ makes it necessary to identify methods for geographical classification of this product. Geographical differentiation of olive oils from a local olive variety from nine distinct locations of a peninsula in the west part of Turkey is investigated by using mid-infrared spectroscopic data and several chemical parameters (total phenol content, fatty acid and phenol profile, total carotene and chlorophyll content and oxidative stability). The best differentiation with respect to geographical origin was obtained with partial least square-discriminant analysis (PLS-DA) of a combination of various chemical parameters. The fatty acid profile also provided good separation of geographic locations and was slightly better than mid-infrared analysis. The best separation was achieved with respect to palmitic, oleic and linoleic acid contents of olive oils. However, mid-infrared spectroscopy with the advantages of being environmentally friendly, cost effective and a fast method could also be used to differentiate monovarietal olive oils with respect to their growing locations by factors such as micro-climates, proximity of regions and position to the sea.
1. Introduction
The unique chemistry of olive oil is associated with its positive contribution to human health. The healthier life of people whose basic diet is Mediterranean type is attributed to foods in this diet; consequently, as a significant part of this diet, the production and consumption of olive oil is becoming more popular.1Each olive variety has its own characteristics and naturally the chemical composition of the olive oils vary depending on the olive variety from which the oil is obtained. Chemical parameters of olive oils are also affected by environmental factors such as soil and climate, harvesting time, agronomic conditions (irrigation regime and fertilization), technological factors such as the extraction system and post-harvest storage conditions. As a result, the presence of many different factors makes it difficult to characterize olive oils using a small number of chemical compounds;2 therefore, large data clusters could provide better differentiation. However, evaluating these data is difficult with univariate statistical methods; for this reason, multivariate statistical methods are applied to numerous variables to obtain meaningful interpretation.3 These statistical techniques such as principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) could be applied to the classification of olive oils with respect to their different characteristics such as olive variety or geographical origin using various chemical or spectroscopic parameters. Classification of olive oils with respect to variety and geographical location is important due to the fact that quality and uniqueness of olive oils could be attributed to specific areas where olives grow; therefore, to ensure the origin and quality of olive oil, two different certification systems were created by the European Union and are known as Protected Designation of Origin (PDO) and Protected Geographical Indication (PGI). These geographical labelling systems attribute the quality of the product to its geographical region, contribute to traceability, help prevent adulteration, add extra value to the product and protect the producer and the consumer at the same time.4
In the present study, olive oil obtained from the Erkence variety, which is almost the only significant olive variety grown in the Karaburun Peninsula of Turkey and its close surrounding area, was investigated. This cultivar has not been studied thoroughly although it has a high oil content and ripens earlier compared to other olive types. Moreover, there is an increasing trend toward the production of olive oils obtained from local monovarietal varieties due to the unique organoleptic characteristics of these varieties. Identifying the chemical characteristics of olive oils from the Karaburun Peninsula could be useful to obtain geographical indication labelling for olive oils produced from this variety.
There are various analytical approaches for classification and authentication studies and these approaches can be mainly grouped into four categories: mass spectroscopy techniques, spectroscopic techniques (IR spectroscopy etc.), separation techniques (HPLC, GC etc.), and others (DNA technology etc.).5 These approaches have their own advantages and disadvantages; therefore, one of the ways of overcoming the weaknesses of each method could be to combine them to increase the mapping power of analytical methods.5 In the literature, there are several studies using different chromatographic techniques6–9 and various spectroscopic10–12 methods that focus on different individual chemical compounds like phenolics, fatty acids and infrared profiles of olive oils to provide successful differentiation with respect to cultivar and/or geographical origin in combination with chemometric methods.
The aim of this study is to investigate the differentiation of olive oils from a local olive variety (Erkence) with respect to its cultivation area (Karaburun Peninsula and its close vicinity) using various chemical parameters, mid-infrared spectroscopy, and their combination. Eventually, this study allows us to investigate the differentiation power of various chemical parameters for a monovarietal olive oil obtained from local olives of a small geographical area.
2. Materials and methods
2.1. Olive oil samples
The olive oil samples were obtained from fully ripened olives in the harvest year of 2012/13 and they were from the same olive cultivar, Erkence. Samples were from various parts of the Karaburun Peninsula of Izmir. There were a total of 54 samples which were divided into nine classes: Eglenhoca (EH) = 7, Karareis (RS) = 2, Karapinar (KP) = 3, Barbaros (BR) = 4, Gulbahce (GB) = 6, Torasan (TR) = 10, Kuscular (KS) = 5, Ozbek (OZ) = 12 and Urla (UR) = 5, according to the growth location of the olives. Geographical coordinates of the locations are provided in Fig. 1 and the approximate distances from sea for these locations are 5.9 km to BR, 0.6 km to TR, 0.25 km to KP, 1 km to GB, 2 km to OZ, 5.7 km to UR, 4.6 km to KS, 1.5 km to EH and 0.8 km to RS.13 Oils were extracted with an industrial scale two phase decanter system (Polat Machinery, Turkey) capable of processing 1.66 tonnes of olive per h and located in Izmir Institute of Technology Campus and Eglenhoca village of Izmir. Samples were kept in glass bottles in a refrigerator and their head spaces were flushed with N2. All the olive oil samples were randomly analyzed.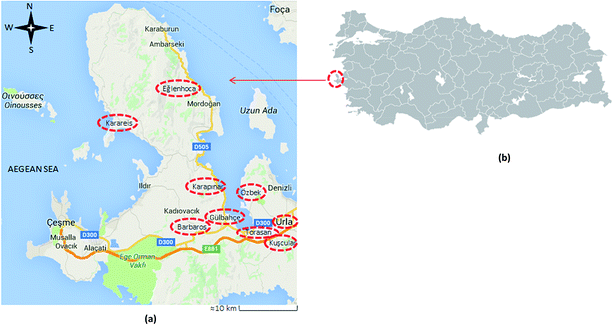 | ||
| Fig. 1 (a) Locations of olive oil samples from Karaburun Peninsula of Turkey and its vicinity: Eglenhoca (EH), Karareis (RS), Karapinar (KP), Barbaros (BR), Gulbahce (GB), Torasan (TR), Kuscular (KS), Ozbek (OZ) and Urla (UR) (with modifications from Google Maps 2015).13 Approximate coordinates: Gulbahce: 38°19′55.6′′N 26°38′36.7′′E, Barbaros: 38°19′21.3′′N 26°34′50.4′′E, Torasan: 38°19′17.3′′N 26°42′35.7′′E, Karapinar: 38°23′10.5′′N 26°37′30.6′′E, Ozbek: 38°21′57.8′′N 26°42′15.9′′E, Urla: 38°19′29.5′′N 26°46′02.3′′E, Kuscular: 38°17′54.1′′N 26°44′59.2′′E, Eglenhoca: 38°32′34.1′′N 26°34′10.6′′E and Karareis: 38°29′04.5′′N 26°25′37.1′′E.13 (b) The location of the Karaburun Peninsula in Turkey.14 | ||
2.2. Chemical reagents
All reagents, used for HPLC and GC analysis were of analytical grade, and were purchased from Riedel-de Haën (Germany), Sigma-Aldrich (Germany) and Merck (Germany).2.3. Oxidative stability
Oxidative stability was determined in terms of hour using Rancimat equipment (873 Biodiesel, Metrohm, Switzerland). The temperature range of this equipment is 50–220 °C with temperature stability of less than 0.1 °C. Three grams of sample was placed inside the glass reaction vessel for the analysis. Deionized water was the carrier medium. For both columns of the equipment, the reaction temperature and air flow rate were kept constant at 120 °C and 20 L h−1, respectively.2.4. Total phenol content (TPC)
The total amount of phenolic compounds was determined using the Folin–Ciocalteu spectrophotometric method.15 Results were calculated in terms of gallic acid (GA) as mg GA kg−1 oil from the gallic acid standard curve. Two measurements were performed for each sample.2.5. Phenolic profiles of olive oils
Phenolic compounds were extracted from the olive oils according to a procedure in the literature.16 Gallic acid was added to the samples as an internal standard.Concentrations of individual phenolic compounds in oil extracts were determined using HPLC (Agilent 1200, USA) having a refractive index (RI) and diode array (DAD) detectors, an auto sampler (ALS G1329A) and a column oven. Analyses were performed with a C18 column (250 × 4 mm, 5 μm, SGE 8211, Australia). Column temperature was constant at 35 °C. Injection volume was 20 μL and the flow rate was set to 1 mL min−1. Mobile phases were water/acetic acid (99.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 v/v) and methanol. Initial concentrations of the mobile phases were 90% for water/acetic acid and 10% for methanol and the concentrations were adjusted according to the following procedure: firstly, the concentration of methanol was raised to 30% in 10 min and kept there for 20 min. Then, the methanol percentage was increased to 40% in 10 min, and kept for another 5 min. This was followed by raising it up to 50% in 5 min, and maintaining it at this concentration for another 5 min. At last, methanol was increased to 60, 70 and 100% in 5 min periods. Finally, initial conditions were obtained at the end of 85 min. Chromatograms were obtained at 280 and 320 nm.
0.2 v/v) and methanol. Initial concentrations of the mobile phases were 90% for water/acetic acid and 10% for methanol and the concentrations were adjusted according to the following procedure: firstly, the concentration of methanol was raised to 30% in 10 min and kept there for 20 min. Then, the methanol percentage was increased to 40% in 10 min, and kept for another 5 min. This was followed by raising it up to 50% in 5 min, and maintaining it at this concentration for another 5 min. At last, methanol was increased to 60, 70 and 100% in 5 min periods. Finally, initial conditions were obtained at the end of 85 min. Chromatograms were obtained at 280 and 320 nm.
Amounts of major phenolic compounds in olive oil samples were determined from calibration curves of commercial standards. Five-point calibration curves for each standard were plotted and the results were expressed in terms of mg kg−1.
2.6. Determination of chlorophyll and carotenoid contents
Chlorophyll and carotenoid concentrations of the samples were determined according to a procedure from the literature.17 The olive oil sample (7.5 g) was dissolved in 25 mL of cyclohexane and the absorbance of the samples at 470 and 670 nm, which are associated with carotene and chlorophyll, respectively, were measured with a UV spectrophotometer (Shimadzu UV-2450 Spectrophotometer, Japan).2.7. Fatty acid profile of olive oils
European Official Methods of Analysis18 was used for fatty acid analysis. Fatty acid profiles of olive oil samples were obtained by a GC (Agilent 6890, Agilent Technologies, USA) having an auto-sampler (Agilent 7863), a split/splitless (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) injector and flame ionization detector (FID). An HP 88 capillary column (Agilent, USA) (100 m × 0.25 mm ID × 0.2 μm) was used. The carrier gas was helium with a 2 mL min−1 constant flow rate. Injection volume was 1 mL. Injection temperature was set to 250 °C while the detector temperature was kept at 280 °C. Oven temperature was 120 °C initially and was maintained there for 10 min then raised with a rate of 3 °C min−1 until it reached 220 °C where it remained for another 5 min. FAME standard peaks were compared with the sample chromatogram and the results were expressed as the percentage of FAME.
50) injector and flame ionization detector (FID). An HP 88 capillary column (Agilent, USA) (100 m × 0.25 mm ID × 0.2 μm) was used. The carrier gas was helium with a 2 mL min−1 constant flow rate. Injection volume was 1 mL. Injection temperature was set to 250 °C while the detector temperature was kept at 280 °C. Oven temperature was 120 °C initially and was maintained there for 10 min then raised with a rate of 3 °C min−1 until it reached 220 °C where it remained for another 5 min. FAME standard peaks were compared with the sample chromatogram and the results were expressed as the percentage of FAME.
2.8. Fourier transform infrared (FTIR) spectroscopy analysis
Mid-infrared spectra (4000–650 cm−1 wavenumber) were obtained with a Perkin Elmer Spectrum 100 FTIR spectrometer (Perkin Elmer Inc., USA) equipped with a deuterated tri-glycine sulphate (DTGS) detector. The instrument has a horizontal attenuated total reflectance (HATR) accessory with a ZnSe crystal. Sixty four scans of each spectrum were obtained. The resolution and scan speed were 4 cm−1 and 1 cm s−1, respectively. Background spectra were collected before each measurement. Measurements were repeated two times.2.9. Statistical analysis
Statistical analyses were performed with SIMCA 13.0.3 software (Umetrics, Sweden). Two multivariate methods, principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA), were used for data analysis.PCA is a well-known unsupervised multivariate technique used to decrease a large number of interrelated variables into a much smaller number of artificial variables which could retain most of the variance in the data set.19 Two statistical parameters, degree of fit (R2) and predictive ability (Q2), are used to evaluate the obtained model performance. At the same time, results of PCA can be given in two complementary plots as scores and loading plots. The score plot indicates how the observations are scattered and which of them are clustered to differentiate principal groupings among observations; in other words, this plot shows similarities and differences between observations. Loading plots, on the other hand, reveal which variables could be associated with which groupings and/or correlations among the observations.20
PLS-DA as a supervised chemometric tool was applied to each data set after PCA was done. It is a linear classification method that combines PLS regression with a classification technique. PLS-DA is based on connecting information in two blocks of variables by maximizing the correlation between them and the main aim is to create a model which is capable of consisting desired classes of observations separately.21
All the chemical parameters were also evaluated by analysis of variance (ANOVA) applying Tukey's test at a 5% significance level with Minitab 16 software (Minitab Inc., State College, USA) in order to analyze if there are any differences between each sample group means.
3. Results and discussion
3.1. Chemical characterization
In order to investigate the chemical characteristics of olive oils from the Erkence variety of Karaburun Peninsula, several important chemical parameters, oxidative stability, chlorophyll and carotenoid content, fatty acid content, total phenol content and phenolic compound profile, were evaluated.Oxidative stability (OS) of all the olive oil groups used in this study is presented in Table 1 and the average value for the overall regions was found to be 1.73 h. It was observed that olive oils belonging to the EH region had the highest average OS (3.36 h) while the rest of the samples had lower values which were close to each other. Statistically, oils from TR, KS and OZ have lower OS compared to oils of EH (Table 1). In the literature, OS values of olive oils obtained from two different Turkish cultivars (Memecik and Ayvalik) were found to be 12.70 h and 7.36 h, respectively22 while in another study, the OS index of olive oils from different regions of Argentina showed a wide variation between 6.7 and 23.3 h.23 These findings were higher than the results of the present study and these OS value fluctuations could be due to the fact that OS is affected by the compositional parameters (fatty acid profile and concentration of minor compounds) as well as post-harvest conditions (post storage temperature, light etc.).23–27 Average concentrations of color pigments, chlorophyll and carotenoid, of olive oils from all studied regions were determined to be 1.96 mg kg−1 oil and 4.20 mg kg−1 oil, respectively. Although univariate statistical analysis shows that there are no significant differences between chlorophyll content belonging to the nine regions, olive oils from the RS region had the highest average of chlorophyll content (2.82 mg kg−1) while the KS region oils had the lowest (1.30 mg kg−1). Tukey test did not indicate any significant difference between regions in terms of the carotenoid content; however, the GB region olive oils had the highest average concentration (5.86 mg kg−1) and the lowest average content was (2.12 mg kg−1) for oils from the TR region (Table 1). According to a study in the literature, olive oil samples obtained from southern and central Italy had total chlorophyll and carotenoid contents in the range of 1.00–26.64 mg kg−1 and 4.19–16.12 mg kg−1 on average, respectively28 and our average values are in the range of this study in the literature. In the current study, no correlation was established between the carotenoid and chlorophyll contents of olive oils.
| EH | RS | KP | BR | GB | TR | KS | OZ | UR | |
|---|---|---|---|---|---|---|---|---|---|
| a Parameters are the averages of all the olive oils belonging to that region ± mean standard deviation. Mean values that do not share a common letter are significantly different (p < 0.05). nd: not determined. 1Oxidative stability, 2chlorophyll, 3carotenoid, 4palmitic acid, 5palmitoleic acid, 6margaric acid, 7stearic acid, 8oleic acid, 9linoleic acid, 10arachidic acid, 11gondoic acid, 12linolenic acid, 13behenic acid, 14lignoceric acid,15saturated fatty acids, 16monounsaturated fatty acids, 17polyunsaturated fatty acids, 18total phenol content, 19hydroxytyrosol, 20tyrosol, 214-hydroxyphenyl acetic acid, 223-hydroxyphenyl acetic acid, 23vanillic acid, 24syringic acid, 25cinnamic acid, 26o-coumaric acid, 272,3-dihydroxybenzoic acid, 28chlorogenic acid, 29caffeic acid, 30vanillin, 31p-coumaric acid, 32apigenin, and 33luteolin. | |||||||||
| 1OS (h) | 3.36a ± 0.49 | 1.60ab ± 1.17 | 1.64ab ± 1.39 | 1.89ab ± 1.96 | 1.86ab ± 1.52 | 1.13b ± 1.25 | 1.14b ± 0.35 | 1.13b ± 0.56 | 1.79ab ± 1.67 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Colour pigments (mg kg −1 ) | |||||||||
| 2CHL | 1.50a ± 0.54 | 2.82a ± 1.94 | 1.76a ± 0.83 | 2.07a ± 1.23 | 2.45a ± 1.03 | 1.35a ± 0.47 | 1.30a ± 0.36 | 1.94a ± 1.09 | 2.45a ± 1.21 |
| 3CRT | 2.68a ± 1.33 | 5.13a ± 5.41 | 4.48a ± 1.70 | 4.50a ± 7.12 | 5.86a ± 3.09 | 2.12a ± 1.10 | 2.81a ± 1.91 | 4.39a ± 2.98 | 5.81a ± 4.37 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Fatty acids (%) | |||||||||
| 4C16:0 | 11.73b ± 0.54 | 13.86a ± 0.45 | 13.06a ± 0.52 | 13.21a ± 0.47 | 13.67a ± 0.91 | 14.01a ± 0.49 | 14.41a ± 0.68 | 13.56a ± 0.42 | 13.97a ± 0.77 |
| 5C16:1 | 0.44c ± 0.14 | 1.14ab ± 0.19 | 0.74abc ± 0.06 | 0.82abc ± 0.17 | 0.72bc ± 0.44 | 0.91ab ± 0.19 | 1.16a ± 0.23 | 0.76bc ± 0.11 | 0.76abc ± 0.15 |
| 6C17:0 | 0.14a ± 0.00 | 0.18a ± 0.04 | 0.12a ± 0.03 | 0.11a ± 0.08 | 0.14a ± 0.03 | 0.13a ± 0.05 | 0.13a ± 0.01 | 0.15a ± 0.01 | 0.18a ± 0.04 |
| 7C18:0 | 2.81bc ± 0.08 | 3.71a ± 0.31 | 2.96abc ± 0.25 | 3.07abc ± 0.40 | 2.97abc ± 0.20 | 2.74c ± 0.18 | 2.76bc ± 0.38 | 2.96abc ± 0.35 | 3.40ab ± 0.06 |
| 8C18:1n9c | 69.15a ± 1.43 | 70.01a ± 1.79 | 70.45a ± 1.49 | 69.81a ± 1.72 | 68.57a ± 0.93 | 68.14a ± 1.75 | 68.91a ± 0.90 | 67.88a ± 1.04 | 67.39a ± 1.21 |
| 9C18:2n6c | 14.11a ± 0.94 | 9.23b ± 1.01 | 10.96ab ± 1.91 | 11.31ab ± 2.56 | 12.14ab ± 1.81 | 12.23ab ± 2.02 | 10.89b ± 1.14 | 12.80ab ± 1.03 | 12.37ab ± 1.48 |
| 10C20:0 | 0.43b ± 0.01 | 0.51ab ± 0.04 | 0.45ab ± 0.01 | 0.45ab ± 0.08 | 0.45ab ± 0.05 | 0.44b ± 0.03 | 0.45ab ± 0.07 | 0.45b ± 0.03 | 0.54a ± 0.09 |
| 11C20:1 | 0.68a ± 0.05 | 0.80a ± 0.07 | 0.69a ± 0.04 | 0.70a ± 0.07 | 0.80a ± 0.10 | 0.82a ± 0.19 | 0.75a ± 0.08 | 0.79a ± 0.21 | 0.80a ± 0.10 |
| 12C18:3n3 | 0.32a ± 0.01 | 0.31a ± 0.03 | 0.32a ± 0.03 | 0.28a ± 0.03 | 0.32a ± 0.06 | 0.34a ± 0.06 | 0.29a ± 0.03 | 0.35a ± 0.15 | 0.32a ± 0.04 |
| 13C22:0 | 0.13a ± 0.00 | 0.13a ± 0.00 | 0.13a ± 0.01 | 0.12a ± 0.10 | 0.13a ± 0.02 | 0.11a ± 0.04 | 0.11a ± 0.01 | 0.13a ± 0.01 | 0.15a ± 0.03 |
| 14C24:0 | ndb | 0.09ab ± 0.01 | 0.08ab ± 0.01 | 0.06ab ± 0.05 | 0.08ab ± 0.06 | 0.10a ± 0.08 | 0.10a ± 0.03 | 0.07ab ± 0.03 | 0.09ab ± 0.07 |
| 15SFA | 15.25b | 18.48a | 16.82ab | 17.05a | 17.47a | 17.54a | 17.98a | 17.33a | 18.33a |
| 16MUFA | 70.27a | 71.94a | 71.88a | 71.33a | 70.08a | 69.87a | 70.82a | 69.43a | 68.94a |
| 17PUFA | 14.43a | 9.58b | 11.30ab | 11.62ab | 12.50ab | 12.60ab | 11.20b | 13.22ab | 12.73ab |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Phenolic compounds (mg kg −1 ) | |||||||||
| 18TPC | 243.68a ± 20.87 | 238.56a ± 60.51 | 266.37a ± 35.72 | 327.42a ± 57.49 | 290.12a ± 106.12 | 301.10a ± 64.17 | 260.60a ± 31.62 | 288.05a ± 67.37 | 283.11a ± 62.74 |
| 19Hxty | 4.44a ± 1.95 | 2.24a ± 1.96 | 1.43a ± 0.76 | 7.71a ± 6.89 | 5.85a ± 10.82 | 5.12a ± 8.06 | 4.65a ± 5.20 | 5.96a ± 8.38 | 3.91a ± 3.84 |
| 20Tyrs | 6.93a ± 2.96 | 4.11a ± 0.46 | 9.36a ± 3.11 | 16.39a ± 10.78 | 8.48a ± 7.96 | 15.21a ± 15.36 | 10.66a ± 7.54 | 11.60a ± 7.68 | 8.64a ± 13.01 |
| 214-Hypa | 0.29a ± 0.07 | 0.64a ± 0.28 | 0.53a ± 0.33 | 0.86a ± 0.57 | 0.48a ± 0.22 | 1.34a ± 1.69 | 0.92a ± 0.27 | 0.83a ± 0.65 | 0.29a ± 0.27 |
| 223-Hypa | 0.57a ± 0.14 | 0.35a ± 0.03 | 0.44a ± 0.13 | 0.72a ± 0.11 | 0.48a ± 0.25 | 0.78a ± 0.61 | 0.57a ± 0.15 | 0.62a ± 0.28 | 0.38a ± 0.23 |
| 23Vna | 0.43a ± 0.11 | 0.79a ± 0.13 | 0.57a ± 0.28 | 1.17a ± 0.46 | 0.58a ± 0.31 | 1.02a ± 0.77 | 1.20a ± 0.25 | 0.99a ± 0.45 | 0.45a ± 0.27 |
| 24Sya | 0.09ab ± 0.04 | 0.21a ± 0.24 | 0.05b ± 0.05 | 0.10ab ± 0.04 | 0.08ab ± 0.06 | 0.09ab ± 0.04 | 0.08ab ± 0.02 | 0.07b ± 0.04 | 0.07ab ± 0.04 |
| 25Cina | 0.04a ± 0.01 | 0.07a ± 0.08 | 0.03a ± 0.04 | 0.07a ± 0.06 | 0.07a ± 0.05 | 0.09a ± 0.13 | 0.12a ± 0.12 | 0.06a ± 0.07 | 0.03a ± 0.04 |
| 26 o-Cou | 0.00a ± 0.01 | 0.08a ± 0.10 | nda | nda | nda | 0.05a ± 0.10 | 0.01a ± 0.02 | nda | nda |
| 272,3-Dhxyb | ndb | ndab | ndb | 0.04a ± 0.05 | ndb | 0.01b ± 0.02 | ndb | ndb | ndb |
| 28Chla | nda | nda | nda | 0.02a ± 0.03 | 0.00a ± 0.01 | 0.01a ± 0.02 | 0.01a ± 0.02 | 0.01a ± 0.02 | nda |
| 29Cfa | 0.03b ± 0.03 | 0.06ab ± 0.03 | 0.04ab ± 0.06 | 0.09ab ± 0.02 | 0.07ab ± 0.03 | 0.12ab ± 0.13 | 0.26a ± 0.21 | 0.17ab ± 0.10 | 0.03b ± 0.02 |
| 30Vnl | 0.12ab ± 0.04 | 0.11ab ± 0.04 | 0.10ab ± 0.03 | 0.13ab ± 0.09 | 0.10ab ± 0.08 | 0.06b ± 0.04 | 0.18ab ± 0.15 | 0.31a ± 0.31 | 0.16ab ± 0.14 |
| 31 p-Cou | 0.18a ± 0.13 | 0.37a ± 0.37 | 0.98a ± 0.55 | 0.96a ± 0.44 | 1.16a ± 0.63 | 1.67a ± 2.33 | 2.39a ± 1.76 | 1.32a ± 0.78 | 0.26a ± 0.23 |
| 32Apig | 0.10b ± 0.25 | 1.27ab ± 0.96 | 2.18ab ± 0.74 | 1.28ab ± 0.55 | 2.10a ± 1.11 | 1.44ab ± 1.66 | 0.74ab ± 0.75 | 1.60ab ± 1.10 | 0.53ab ± 0.47 |
| 33Lut | 0.84a ± 0.66 | 0.04ab ± 0.01 | 0.18ab ± 0.19 | 0.03b ± 0.04 | 0.15b ± 0.18 | 0.08b ± 0.12 | 0.59ab ± 0.71 | 0.17b ± 0.30 | 0.01b ± 0.03 |
Fatty acid profiles of all olive oil samples are presented in Table 1. The distribution of fatty acids in olive oil samples was in the range of the European Standard for Olive Oils and Olive Pomace Oils.29 Individual fatty acid content of the samples from different areas is quite close to each other. According to the literature, fatty acid composition is mainly affected by genotype (cultivar).30 In the present study, all olive oil samples belong to the same olive cultivar (Erkence); therefore, the similarity in the fatty acid content is expected. There are very few studies in the literature about the Erkence type of olive oil and according to one of these studies olive oil from the Erkence variety obtained in two different harvest years had an average oleic acid content of 66.44% and 63.57%.31 In the present study, the oleic acid content (68.92%) in terms of average sum of the whole Karaburun Peninsula region was higher compared to the previous study.31 According to the Tukey test there is no statistically significant difference between the oleic acid content of olive oils belonging to different regions of the Karaburun Peninsula. Another important polyunsaturated fatty acid (PUFA), linoleic acid, was found in the range of 7.87 to 15.13% with an average value of 11.78%. All the fluctuations observed between the years could be attributed to the climatic conditions and the differences in the cultivation areas of olives.
The total phenol contents (TPC) of olive oils is also listed in Table 1 and it is observed that the olive oils obtained from the BR region possessed the highest TPC (327.42 mg GA kg−1 oil) whereas the RS region had the lowest (238.56 mg GA kg−1 oil) although the difference is not statistically significant. According to a study in the literature, Erkence olive oil had an average TPC of 333.37 mg GA kg−1 oil and 356.65 mg GA kg−1 oil in different harvest years.21 In the present study, the average of the whole Karaburun Peninsula region's TPC was determined as 277.67 mg GA kg−1 which was lower compared to a previous study in the literature. TPC is affected by many factors like environment, harvest year and geographical location.21
Significant phenolic compounds for olive oils from the Erkence type were found as hydroxytyrosol, tyrosol, 4-hydroxyphenyl acetic acid, 3-hydroxyphenyl acetic acid, vanillic acid, p-coumaric acid and apigenin. According to a study in the literature, Erkence olive oil obtained from Izmir was classified with another 5 cultivars and all the cultivars were rich in terms of hydroxytyrosol, tyrosol, vanillic acid, p-coumaric acid, cinnamic acid, luteolin and apigenin.21 In the present study, 4-hydroxyphenyl acetic acid and 3-hydroxyphenyl acetic acid were determined in significant amounts and they are the distinguishing compounds from the past study. Hydroxytyrosol (1.43–7.71 mg kg−1) and tyrosol (4.11–16.39 mg kg−1) were in higher amounts than the previous study while apigenin, luteolin and cinnamic acid concentrations were lower. 4-Hydroxphenyl acetic, vanillic and p-coumaric acids were detected in significantly higher amounts. The rest of the phenolic compounds (2,3-dihydroxybenzoic acid, caffeic acid and vanillin) had similar concentrations with the previous work.21 Although univariate statistical analysis of the data did not result in significant differentiation between regions for some parameters, multivariate analysis which takes into account multiple parameters at the same time indicated better separation for various regions.
3.2. Differentiation of olive oils
Discrimination of olive oils obtained from the Erkence variety from different parts of the Karaburun Peninsula was performed by using 11 fatty acids, 15 phenolic compounds and mid-infrared spectra separately as data sets. In addition, fatty acids, phenolic compounds and TPC, chlorophyll and carotenoid content and OS were used together to generate another data set. Although the number of samples (54) could be considered as moderate compared to other studies, this sample size is representative of olive oils produced in this small area. Statistics for all models are shown in Table 2. First, PCA was used in evaluating 54 observations belonging to 9 regions and score plots were generated for each parameter. It was observed that data belonging to some regions are too scattered; therefore, these regions are excluded and PLS-DA models are constructed for the rest.| Models overall | PLS-DA models | |||||
|---|---|---|---|---|---|---|
| Statistical | FTIR | Combination of several chemical parameters | Fatty acids | Phenolic compounds | ||
| Parameters | Model I | Model II | Model III | Model IV | ||
| PC | 4 | 4 | 5 | 6 | 4 | 3 |
| Number of samples | 24 | 17 | 24 | 17 | 24 | 24 |
| R 2 | 0.94 | 0.97 | 0.87 | 0.96 | 0.72 | 0.61 |
| Q 2 | 0.26 | 0.43 | 0.50 | 0.66 | 0.48 | 0.35 |
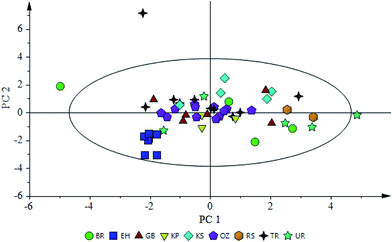 | ||
| Fig. 2 Score plot of a PCA model constructed with a fatty acid profile of olive oils from different regions. | ||
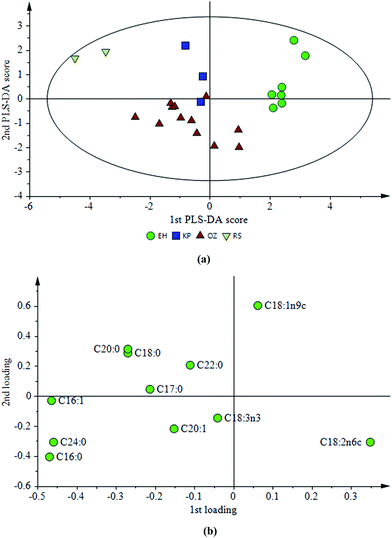
The overall PLS-DA model constructed with a fatty acid profile had 24 observations and possesses four components explaining 72% of the total variation with 48% prediction ability (Table 2). From the score plot of the PLS-DA model (Fig. 3a), it could be seen that EH, KP, OZ, and RS regions are separated from each other successfully, and especially the EH and RS regions are quite different than the rest of the group. The reason for that successful separation could be that, while the EH region is located on the east side of the peninsula, RS is on the west part. Therefore, olives cultivated in these areas are exposed to different sub-climates although these locations are not very far from each other. The same argument also holds true for the OZ region which is located not in the peninsula but in very close vicinity. This area is not exposed to open sea winds as in the RS region. KP is, on the other hand, just across the OZ region and again in a more sheltered location compared to the EH and RS regions. In terms of the fatty acid profile, KP and OZ oils are closer in the score plot (Fig. 3a). The loading plot for this model is also presented in Fig. 3b and this plot shows which fatty acids are responsible for differentiation. In this case; linoleic (C18:2n6c), palmitic (C16:0) and oleic acids (C18:1n9c) are located far away from the origin which indicates high differentiation power. The EH region is differentiated from others due to its low palmitic acid content while a low amount of oleic acid for OZ region oils caused a separation. Furthermore, olive oils from the RS region are distinguished from the rest by having a low linoleic acid content. In the literature, it has been proven that these three fatty acids have a high classification power and also it was indicated that the effect of cultivar is the most dominant factor in olive oil classification according to fatty acid composition.8 In addition, it was found that geographical location could have a minor but well defined effect on classification.8 In the present study, even if most of the parameters, such as the olive variety, extraction method, maturation stage of olives and harvest year, are the same for each olive oil sample except the growing locations of the olives, a clear differentiation for some locations is still observed in terms of the fatty acid profile.
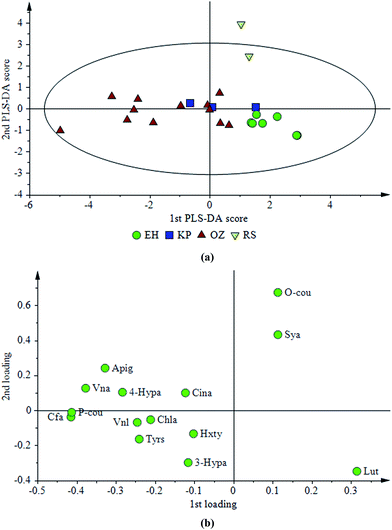 | ||
| Fig. 4 (a) Score and (b) loading plots of a PLS-DA model constructed with phenolic profiles of olive oils from different regions. | ||
Studies investigating the effect of geographical locations have been mostly performed with different cultivars in the literature but only one variety is investigated in the present study. In the literature, the relation between the amount of phenolic compounds and geographical origin for the same olive type was investigated for a single variety of olive cultivated in different parts of southern Catalonia.32 Results of this study32 showed that the phenolic composition of olive oil is highly dependent on geographical area; therefore, phenolic composition could be used for classification studies even for the samples belonging to the same cultivar but grown in different areas. In the present study, three areas (RS, EH and OZ) were differentiated successfully and the reason for the lower differentiation power of phenolic compounds could be the close proximity of the defined regions (Fig. 1).
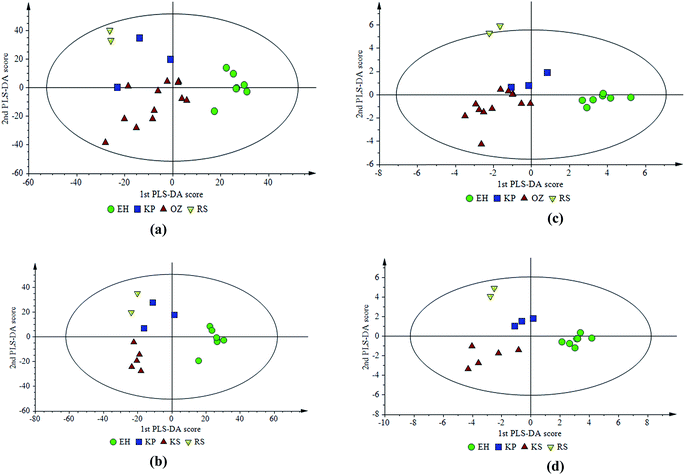 | ||
| Fig. 5 PLS-DA score plots created using (a) and (b) infrared profiles, (c) and (d) various chemical parameters for the defined regions. | ||
In addition, two different PLS-DA models of combinations of various chemical parameters, including the fatty acid profile, phenolic compounds and TPC, chlorophyll and carotenoid content, and oxidative stability, were also constructed as models III and IV corresponding to Fig. 5c and d, respectively with the same two clusters mentioned above to compare with infrared spectroscopic models (I and II). Statistics of the PLS-DA models are provided in Table 2.
First two PCs of the first PLS-DA model (I) shown in Fig. 5a (R2 = 0.94 and Q2 = 0.26) constructed with the FTIR profile can differentiate EH and RS regions successfully while the separation of the KP and OZ regions is not that clear. On the other hand, the model (III) constructed by various chemical parameters (R2 = 0.87 and Q2 = 0.50) is similar and oils from the same regions are clustered better (Fig. 5c). Another observation set was also used for further comparison of mid-infrared spectroscopic and chemical parameter data for geographical differentiation. The first two PCs of the second model (II) shown in Fig. 5b created by the infrared profile resulted in R2 = 0.97 and Q2 = 0.43 while R2 = 0.96 and Q2 = 0.66 were obtained when combinations of several chemical parameters were used as the fourth model (IV) data set (Fig. 5d). PLS-DA could differentiate all the defined regions successfully by using both FTIR data (Fig. 5b) and a combination of various chemical parameters (Fig. 5d). Better clustering with chemical parameters was obtained again with this data set. A comparison of the score plots obtained with mid-IR data and a combination of chemical parameters for two data sets (Fig. 5) reveals that very similar differentiation patterns in terms of geographical locations could be obtained by using these data sets. From the statistical values of the overall models presented in Table 2, it could be seen that two models III and IV (Fig. 5c and d) created by a combination of various chemical parameters have a higher Q2 value than infrared profile based models I and II (Fig. 5a and b) meaning a better differential power on distinct areas.
According to a study in the literature, six geographically close registered designation of origin regions (RDOs) could be satisfactorily classified by predicting fatty acids and triacylglycerols from FTIR spectra.33 In order to examine the variety effect, a comparison of two Turkish olive varieties from two different areas of Izmir was performed by using two different data sets of fatty acid and FTIR profiles and it was observed that separation was successful to some degree by FTIR whereas differentiation by fatty acid profile was better compared to FTIR.31 In the present study, similar conclusions are also obtained. When the PLS-DA models generated either by mid-infrared or fatty acid profiles are examined (Fig. 5a and 3a) it could be concluded that the fatty acid profile possesses a slightly better differentiation power than the FTIR profile even in a narrow plantation area. With the present study, it is also observed that a combination of various chemical parameters produced even better geographical separation than using the fatty acid profile alone since model statistical values are better for these models (Table 2).
4. Conclusions
In the present study, the aim was to differentiate olive oils obtained from the Erkence variety olive from different parts of the Karaburun Peninsula using several chemical parameters and mid-infrared spectroscopic data. The best separation in terms of growing location was obtained with a combination of several chemical parameters. However, mid-infrared and fatty acid profiles separately also resulted in similar differentiation. Since FTIR spectroscopy is a rapid and easy-to-use technique it could be preferred for this type of application. In addition, it was observed that olive oils from the same variety of oils could be separated depending on their growing locations even in a small area depending on their position in the peninsula (e.g. east or west, open to the sea or in a more protected area). However, other factors such as the proximity to the sea and micro-climates might also affect the geographical differentiation of the olive oils.Acknowledgements
We would like to thank Biotechnology and Bioengineering Research Center and Environmental Research Center of Izmir Institute of Technology for their assistance in HPLC and GC analyses.References
- J. R. Morelló, M. J. Motilva, M. J. Tovar and M. P. Romero, Food Chem., 2004, 85, 357–364 CrossRef.
- R. Aparicio and G. Luna, Eur. J. Lipid Sci. Technol., 2002, 104, 614–627 CrossRef CAS.
- O. Uncu and B. Ozen, LWT--Food Sci. Technol., 2015, 63, 978–984 CrossRef CAS.
- B. A. Babcock and R. Clemens, Geographical indications and property rights: protecting value-added agricultural products, MATRIC Briefing Paper, 04-MBP 7, Iowa State University, Ames, IA, 2004 Search PubMed.
- D. M. A. M. Luykx and S. M. Van Ruth, Food Chem., 2008, 107, 897–911 CrossRef CAS.
- F. Aranda, S. Gómez-Alonso, R. M. R. Del Álamo, M. D. Salvador and G. Fregapane, Food Chem., 2004, 86, 485–492 CrossRef CAS.
- D. Ollivier, J. Artaud, C. Pinatel, J. P. Durbec and M. Guérère, Food Chem., 2006, 97, 382–393 CrossRef CAS.
- M. D'Imperio, G. Dugo, M. Alfa, L. Mannina and A. L. Segre, Food Chem., 2007, 102, 956–965 CrossRef.
- D. Alkan, F. Tokatli and B. Ozen, J. Am. Oil Chem. Soc., 2012, 89, 261–268 CrossRef CAS.
- A. Bendini, L. Cerretani, F. Di Virgilio, P. Belloni, M. Bonoli-Carbognin and G. Lercker, J. Food Qual., 2007, 30, 424–437 CrossRef CAS.
- M. Casale, C. Casolino, P. Oliveri and M. Forina, Food Chem., 2010, 118, 163–170 CrossRef CAS.
- F. Longobardi, A. Ventrella, C. Napoli, E. Humpfer, B. Schütz, H. Schäfer, M. G. Kontominas and A. Sacco, Food Chem., 2012, 130, 177–183 CrossRef CAS.
- Google Maps, https://www.google.com.tr/maps/, accessed November 2015.
- A. G. Baydin, “BlankMapTurkeyProvinces” – Self drawn from scratch, based on data from official Turkish motorway network map. Licensed under CC BY-SA 3.0 via Commons, https://commons.wikimedia.org/wiki/File:BlankMapTurkeyProvinces.png#/media/File:BlankMapTurkeyProvinces.png, accessed November 2015.
- G. Montedoro, M. Servili, M. Baldioli and E. Miniati, J. Agric. Food Chem., 1992, 40, 1571–1576 CrossRef CAS.
- M. Brenes, A. García, P. García, J. J. Rios and A. Garrido, J. Agric. Food Chem., 1999, 47, 3535–3540 CrossRef CAS PubMed.
- M. I. Mínguez-Mosquera, L. Rejano-Navarro, B. Gandul-Rojas, A. H. Sánchez Gómez and J. Garrido-Fernández, J. Am. Oil Chem. Soc., 1991, 68, 332–336 CrossRef.
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis, Off. J. Eur. Commun., 1991, L248, 1–83.
- I. T. Jolliffe, Principal component analysis, Springer-Verlag, New York, NY, 2nd edn, 2002 Search PubMed.
- M. R. Euerby and P. Petersson, J. Chromatogr. A, 2003, 994, 13–36 CrossRef CAS PubMed.
- D. Ocakoglu, F. Tokatli, B. Ozen and F. Korel, Food Chem., 2009, 113, 401–410 CrossRef CAS.
- M. Kıralan and A. Bayrak, Int. J. Food Prop., 2013, 16, 649–657 CrossRef.
- L. N. Ceci and A. A. Carelli, J. Am. Oil Chem. Soc., 2007, 84, 1125–1136 CrossRef CAS.
- F. Gutiérrez, B. Jímenez, A. Ruíz and M. A. Albi, J. Agric. Food Chem., 1999, 47, 121–127 CrossRef.
- E. Gimeno, A. I. Castellote, R. M. Lamuela-Raventós, M. C. De la Torre and M. C. López-Sabater, Food Chem., 2002, 78, 207–211 CrossRef CAS.
- H. Dıraman and H. Dibeklioglu, J. Am. Oil Chem. Soc., 2009, 86, 663–674 CrossRef.
- J. Velasco and C. Dobarganes, Eur. J. Lipid Sci. Technol., 2002, 104, 661–676 CrossRef CAS.
- D. Giuffrida, F. Salvo, A. Salvo, L. Cossignani and G. Dugo, Food Chem., 2011, 124, 1119–1123 CrossRef CAS.
- Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive and olive pomace oils and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff, Off. J. Eur. Commun., 2002, L128, 8–28.
- S. Lanteri, C. Armanino, E. Perri and A. Palopoli, Food Chem., 2002, 76, 501–507 CrossRef CAS.
- G. Gurdeniz, B. Ozen and F. Tokatli, Eur. Food Res. Technol., 2008, 227, 1275–1281 CrossRef CAS.
- A. Bakhouche, J. Lozano-Sánchez, R. Beltrán-Debón, J. Joven, A. Segura-Carretero and A. Fernández-Gutiérrez, Food Res. Int., 2013, 50, 401–408 CrossRef CAS.
- O. Galtier, Y. Le Dréau, D. Ollivier, J. Kister, J. Artaud and N. Dupuy, Appl. Spectrosc., 2008, 62, 583–590 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |