Open Access Article
Open Access ArticleRapid detection of toxic compounds in tobacco smoke condensates using high-resolution 1H-nuclear magnetic resonance spectroscopy†
Jana
Ticha
* and
Christopher
Wright
British American Tobacco, Research and Development, Southampton, UK. E-mail: jana_ticha@bat.com
First published on 10th August 2016
Abstract
In 2012, the FDA Tobacco Products Scientific Advisory Committee published a list of 93 harmful and potentially harmful constituents (HPHCs) of tobacco products and tobacco smoke. This list includes many of the “Hoffmann analytes”—the most frequently cited substances regarding the negative health effects of cigarette smoking. Proposed changes to US tobacco product regulation require reporting of HPHC concentrations in smoke. Fit-for-purpose analytical methods for measurement of HPHCs are a priority for regulatory agencies and the tobacco industry, but the chemical diversity of these substances dictates labor-intensive analyses by established techniques. Here, a semi-quantitative analysis of organic compounds on the Hoffmann list was developed using high-resolution proton nuclear magnetic resonance (HR 1H NMR). The data acquisition protocol was validated and used to build a database of analytes in methanolic tobacco smoke condensate (TSC) of Kentucky 3R4F research cigarettes. Among 33 Hoffmann analytes amenable to NMR measurement, 20 were detected directly in TSC. For the 13 undetected substances, fortification experiments were conducted to identify the concentrations at which they were detectable. Among 34 further FDA HPHCs analyzed, 13 were detectable in 3R4F TSC via overspiking experiments. The chemical shifts of these 13 compounds plus the 20 Hoffman analytes establish a database of 33 smoke toxicants measureable in a single NMR analysis. This approach is compatible with standardized smoke collection procedures and allows rapid and consistent measurement of the selected substances in TSC. It will facilitate the chemical evaluation of large numbers of TSC samples with relatively high throughput and acceptable results reproducibility.
Tobacco smoke is a complex and dynamic aerosol consisting of liquid droplets suspended in a mixture of gases and semi-volatiles.1 A series of complex overlapping processes, including combustion, pyrolysis, pyrosynthesis, distillation, sublimation, condensation, filtration and elution, occur during the generation of tobacco smoke.2 Smoke is drawn through the filter-tip of the ignited cigarette as mainstream smoke, and is also released from the smoldering tip of a cigarette as sidestream smoke.3–5 More than 7000 compounds have been reported in tobacco and over 4700 have been identified in mainstream smoke,6–11 while some researchers have suggested that cigarette smoke may comprise more than 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 constituent chemicals.12 The substances identified in mainstream cigarette smoke have varying physical and chemical properties and include neutral gases, carbon and nitrogen oxides, amides, imides, lactams, carboxylic acids, lactones, esters, aldehydes, ketones, alcohols, phenols, amines, N-nitrosamines, N-heterocycles, aliphatic hydrocarbons, monocyclic and polycyclic aromatic hydrocarbons, nitriles, anhydrides, carbohydrates, ethers, nitro compounds and metals.13
000 constituent chemicals.12 The substances identified in mainstream cigarette smoke have varying physical and chemical properties and include neutral gases, carbon and nitrogen oxides, amides, imides, lactams, carboxylic acids, lactones, esters, aldehydes, ketones, alcohols, phenols, amines, N-nitrosamines, N-heterocycles, aliphatic hydrocarbons, monocyclic and polycyclic aromatic hydrocarbons, nitriles, anhydrides, carbohydrates, ethers, nitro compounds and metals.13
Researchers and public health organizations have proposed numerous lists of substances in mainstream smoke that should be prioritized with regard to human health, including 44 substances subsequently named the ‘Hoffmann analytes’.7 Hoffmann analytes include substances prioritized by World Health Organisation (WHO) Framework Convention on Tobacco Control, i.e. acetaldehyde, acrolein, carbon monoxide, benzene, 1,3-butadiene, formaldehyde, N-nitrosonornicotine (NNN), N-nitrosonornicotine ketone (NNK), benzo[a]pyrene (B[a]P) and nicotine. In June 2009, the Family Smoking Prevention and Tobacco Control Act became law in the United States and assigned authority to the FDA to regulate the manufacture, distribution and marketing of tobacco products to protect public health.14 It also imposed obligations on the US tobacco industry to measure and report chemical constituents of tobacco products and cigarette smoke. In 2012, the FDA Tobacco Products Scientific Advisory Committee published a list of 93 harmful and potentially harmful constituents (HPHCs) defined as chemical compounds present in tobacco products or tobacco smoke that are known to cause, or potentially to cause, serious illnesses such as cancer, cardiovascular diseases and chronic obstructive pulmonary disease.15 The FDA HPHC and Hoffman analytes lists overlap in 35 substances. The Hoffmann analytes not included in the current HPHCs list are 3-aminobiphenyl, butyraldehyde, hydroquinone, N-nitrosoanabasine (NAB), N-nitrosoanatabine (NAT), nitric oxide, nicotine-free-dry-particulate-matter (NFDPM, “tar”), pyridine and resorcinol.
Under section 904 of the Tobacco Control Act, US tobacco product manufacturers and importers have been obliged to report the concentration of a subset of 20 HPHCs in tobacco products or tobacco smoke by brand since June 2012, and will have to report the remaining HPHCs in due course.16 The development of appropriate analytical methods for the measurement of HPHCs in tobacco and smoke is therefore a priority for both regulatory agencies and the tobacco industry, but the diversity of these chemical substances currently dictates a labor-intensive analysis by various specialized techniques.9 For example, current determination of the 44 Hoffmann toxicants typically requires 14 separate analyses, including HPLC/UV, HLPC/MS, GC/MS, IC and ICP/MS. Furthermore, the established methods are fully targeted and cannot provide any additional information, such as data on an additional member of a homologous series, without modification or repeat analysis.17–26 Moreover, the FDA list may be reviewed and altered over time, requiring further modifications to established methods.
Recently, attention has been turning from the targeted measurement of cigarette smoke constituents to chemical screening that can increase the characterization of tobacco smoke. This latter strategy provides significantly more comprehensive information than the targeted measurement of specific groups of substances and generates data that can be retrospectively re-analyzed (or ‘mined’) to provide information in the light of new knowledge (e.g., to determine whether a previously ‘insignificant’ substance was present in historical samples). Recent screens of tobacco smoke have applied predominantly chromatographic approaches that limit the number and range of substances discernible and thereby provide only partial information.21,27 While it is likely that no single analytical technique will be able to measure all of the constituents of cigarette smoke, one that is capable of measuring a large homologous series of multiple compound classes may offer the best approach to tobacco smoke screening.
High-resolution (HR) proton nuclear magnetic resonance (1H NMR) spectroscopy is a comprehensive non-destructive orthogonal technique for both non-targeted and targeted analysis of substances with a wide range of physico-chemical properties. It provides information about the physical and chemical properties of atoms by exploiting the capability of certain nuclei to absorb and emit energy in response to radiofrequency perturbation in the presence of a static magnetic field (B0). A key feature of the NMR spectrum is that the signals arising from a molecule are resolved on the frequency axis (chemical shift). The overall NMR pattern of a mixture is characterized by the sum of all responses relating to each individual substance in the mixture. Thus, within a single NMR measurement it is possible to gain information on the substances present in the mixture at one time, and the data can be further and retrospectively interrogated with regard to additional substances of interest. NMR is also quantitative, because the areas of the signals are directly proportional to the number of active nuclei that give rise to them. Although NMR is generally perceived as less sensitive than other spectroscopic techniques, it is being continually improved by technological developments such as the maximum available magnetic field strength and the application of cryoprobes, which have facilitated detection down to the micromolar range.28–30
HR NMR has several key advantages. It is a non-destructive technique and does not normally require time-consuming sample preparation steps. It is independent of chromatographic methods but complementary to them, and facilitates the confirmation of other spectroscopically derived data. It has the potential to screen the composition of liquid-state samples for a wide range of chemical classes. Since its inception in the 1950s, HR 1H NMR has emerged as an essential tool for chemical research and quality control in the fields of chemistry, biochemistry, physics and medicine.31–35
For example, it has been successfully used to detect unknown contaminants in potable water,36 carbonated37 and alcoholic38,39 beverages. In particular, Charlton et al.36 demonstrated that NMR spectroscopy is an ideal technique for the non-targeted detection of unknown contaminants, showing that mixtures of pesticides, industrial solvents, toxins and explosives could be identified under the same experimental conditions. Lachenmeier et al.40 highlighted a need for non-targeted screening methods in food industry and potential of NMR as routine analytical tool for both, non-targeted and targeted methods. Advantages of NMR were also reported in the analysis of electronic cigarettes (e-cigarettes), for example Hahn et al. used NMR for the analysis of several components of e-cigarette liquids to estimate the risk of consumer exposure.41
Of the 44 Hoffmann analytes that must be measured in cigarette smoke in certain jurisdiction, 33 are indicated as amenable to NMR analysis. However, few studies have applied NMR to cigarette smoke measurement, and those published focus on mainstream smoke particulate matter.42 Pankow et al. published studies concerning the ratio of protonated nicotine and the effect of protonated nicotine formation on pH in particulate matter of selected commercial and reference cigarettes43 and also in the Eclipse “cigarette” product that heats the tobacco instead of burning it by using a carbon rod.44
The aim of the present study was therefore to demonstrate the applicability of HR 1H NMR spectroscopy to the screening and quantification of selected Hoffmann substances and other HPHCs in mainstream tobacco smoke by providing not only a rapid alternative to chromatographic techniques but also a confirmatory spectroscopic technique.
Experimental
Materials
Glass fiber filter pads (44 mm; Cambridge Filter Pads, CFP) were purchased from Borgwaldt KC (Hamburg, Germany). 3R4F reference cigarettes were obtained from the Center for Tobacco Reference Products (University of Kentucky, USA). The CORESTA Monitor test piece CM6 was acquired from Borgwaldt KC. The main characteristics of the two test cigarettes are summarized in Table 1.| Parameter | Mean value (mg per cigarette) | |
|---|---|---|
| 3R4F | CM6 | |
| a Approved monitor no. 6 (CM6). Abbreviations: TPM, total particulate matter; NFPDM, nicotine-free dry particulate matter (TPM with nicotine and water subtracted; ‘tar’). | ||
| Weight | 1060 | 974 |
| TPM45 | 11.0 | 17.54 |
| Nicotine46 | 0.73 | 1.39 |
| NFDPM45 | 10.27 | 14.28 |
| CO47 | 12.0 | 14.83 |
| Puff count48 | 9.0 | 9.15 |
Chemicals
Reference materials were purchased from Cambridge Isotope Laboratories (USA), Sigma Aldrich (UK) and LGC Standards (UK). The full list of 67 chemicals, their purities and CAS numbers is included in ESI Table S1.†Preparation of whole tobacco smoke condensates
The test cigarettes 3R4F and CM6, and CFPs were conditioned according to ISO 3402 (22 ± 1 °C and 60 ± 3% relative humidity for a minimum of 48 hours but not exceeding 10 days) to ensure their consistency.49 One CM6 and five 3R4F smoke samples were generated using a 20-port linear SM450 smoke machine (Cerulean, UK) with each port connected to two Dreschel traps (impingers) connected in series. For both 3R4F and CM6, five cigarettes were smoked under the ISO smoking regime.48 The mainstream cigarette smoke was drawn through a 44 mm CFP and the two impingers, each containing 10 ml of deuterated methanol and placed in isopropyl alcohol/dry ice slurry to maintain the temperature of the tobacco smoke condensate (TSC) below −70 °C. After smoking was complete, the CFP was extracted using 20 ml of deuterated methanol and combined with the Dreschel trap contents to give a total volume of 40 ml (Fig. 1). Care was taken to minimize the risk of evaporative loss of volatile substances by keeping the TSC extracts at −70 °C during extraction, storage and transport. The concentration of matrix in the extracts was therefore equivalent to one cigarette per 8 ml of deuterated methanol.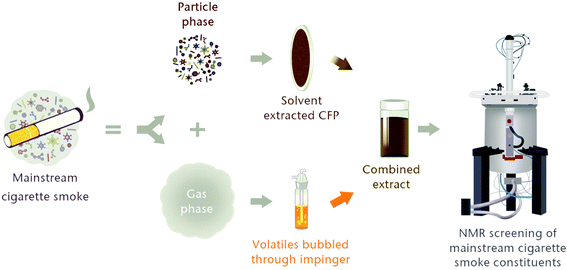 | ||
| Fig. 1 Sample generation workflow. Particulate phase collected on a CFP was extracted and combined with the content of two impingers that were kept at approximately −70 °C. The final extract was stored at −70 °C to minimize the loss of volatile substances. Adapted from Adamson et al.50 | ||
Study design
The study design comprised two investigation stages, each based on a different approach. In the first stage (“profile matching”), the NMR spectra of reference materials were compared with the NMR spectrum of the 3R4F TSC extract. In the second stage, the matrix was overspiked with target analytes to confirm their presence in the TSC. These data were used for the database and were used to develop the NMR protocol. The analytical method was then validated using 3R4F TSC and tested using the CORESTA monitor test piece CM6.Preparation of standard solutions
The detection of each of the targeted substances (Hoffmann analytes in the first part of the study and HPHCs from the FDA list in the second part of the study) in the TSC matrix required standard solutions of these substances, which were prepared by dissolving a known concentration of the given analyte in deuterated methanol. A series of standards was prepared and their spectra acquired by NMR for each of the two investigation stages. The standards used for the profile matching of Hoffman analytes were dissolved at a concentration anticipated for the given analyte in the smoke from a single cigarette (Table 2) and ranged from 1 to 200 mg l−1. The concentration range for the standards of HPHCs was between 3 and 100 mg ml−1. Trimethylsilylpropanoic acid (TSP) was added at a final concentration of 0.1 mM to both standard solutions and TSC extracts as an internal standard to reference the chemical shifts.| Hoffmann analyte | MW (g mol−1) | Abundance in 3R4F51 | Approximate abundancea (g per cig.) | Expected concentration in TSCb (mg l−1) | Concentration added stdc (mg l−1) | |
|---|---|---|---|---|---|---|
| a Approximate abundance of these substances in the smoke of one cigarette expressed in grams. b Expected concentration in the 3R4F TSC expressed in mg l−1. c Concentration of the standard solutions expressed in mg l−1. | ||||||
| Nicotine | 162.23 | 0.73 | mg per cig | 7 × 10−4 | 125.09 | 156.39 |
| Acetaldehyde | 44.05 | 469 | μg per cig | 5 × 10−4 | 125.07 | 156.34 |
| Isoprene | 68.12 | 347 | μg per cig | 3 × 10−4 | 62.55 | 78.2 |
| Acetone | 58.08 | 199 | μg per cig | 2 × 10−4 | 12.51 | 15.62 |
| 1,3-Butadiene | 54.09 | 30.5 | μg per cig | 3 × 10−5 | 6.26 | 7.84 |
| Acrolein | 56.06 | 52.2 | μg per cig | 5 × 10−5 | 6.25 | 7.79 |
| Toluene | 92.14 | 64.5 | μg per cig | 6 × 10−5 | 6.26 | 7.83 |
| Catechol | 110.1 | 38.9 | μg per cig | 4 × 10−5 | 6.25 | 7.82 |
| Hydroquinone | 110.11 | 31.1 | μg per cig | 3 × 10−5 | 6.25 | 7.82 |
| Formaldehyde | 30.03 | 25 | μg per cig | 3 × 10−5 | 1.25 | 1.56 |
| Acrylonitrile | 53.06 | 10.7 | μg per cig | 1 × 10−5 | 1.25 | 1.54 |
| Propionaldehyde | 58.08 | 45 | μg per cig | 5 × 10−5 | 1.25 | 1.57 |
| Crotonaldehyde | 70.09 | 10.7 | μg per cig | 1 × 10−5 | 1.25 | 1.54 |
| Butyraldehyde | 72.11 | 33.6 | μg per cig | 3 × 10−5 | 1.25 | 1.59 |
| 2-Butanone | 72.11 | 52.4 | μg per cig | 5 × 10−5 | 1.25 | 1.59 |
| Benzene | 78.11 | 43.4 | μg per cig | 4 × 10−5 | 1.25 | 1.56 |
| Pyridine | 79.1 | 4.5 | μg per cig | 5 × 10−6 | 1.25 | 1.58 |
| Phenol | 94.11 | 7.6 | μg per cig | 8 × 10−6 | 1.25 | 1.6 |
| Styrene | 104.15 | 4.2 | μg per cig | 4 × 10−6 | 1.25 | 1.56 |
| o-Cresol | 108.14 | 2.49 | μg per cig | 2 × 10−6 | 1.25 | 1.51 |
| m-Cresol | 108.14 | 2.09 | μg per cig | 2 × 10−6 | 1.25 | 1.51 |
| p-Cresol | 108.14 | 4.69 | μg per cig | 5 × 10−6 | 1.25 | 1.51 |
| Resorcinol | 110.1 | 0.78 | μg per cig | 8 × 10−7 | 0.13 | 0.15 |
| Quinoline | 129.16 | 0.27 | μg per cig | 3 × 10−7 | 0.13 | 0.15 |
| NNN | 177.2 | 113.5 | ng per cig | 1 × 10−7 | 7 × 10−3 | 7 × 10−3 |
| NAT | 189.21 | 125.4 | ng per cig | 1 × 10−7 | 1 × 10−3 | 100.09 |
| NNK | 207.23 | 103.6 | ng per cig | 1 × 10−7 | 1 × 10−3 | 100.09 |
| NAB | 191.23 | 14.1 | ng per cig | 1 × 10−8 | 1 × 10−3 | 100.01 |
| 1-Aminonaphthalene | 143.19 | 13.6 | ng per cig | 1 × 10−8 | 1 × 10−3 | 1 × 10−3 |
| 2-Aminonaphthalene | 143.19 | 7.7 | ng per cig | 8 × 10−9 | 1 × 10−3 | 1 × 10−3 |
| 3-Aminobiphenyl | 169.22 | 1.93 | ng per cig | 2 × 10−9 | 2 × 10−3 | 2 × 10−3 |
| 4-Aminobiphenyl | 169.22 | 1.15 | ng per cig | 1 × 10−9 | 1 × 10−4 | 100.01 |
| B[a]P | 252.31 | 6.22 | ng per cig | 6 × 10−9 | 1 × 10−3 | 100.17 |
Overspiking experiments
For the overspiking experiments, standard solutions of the target analytes were prepared at concentrations feasible to observe an increase of 10% in the NMR responses for the given analyte. This general criterion proved to be effective to allow the identification of most of the analytes. However, for some analytes present at levels below the detection limit, more concentrated standard solutions were required.Standards for 22 Hoffmann analytes were prepared in deuterated methanol at a concentration 1.25 times higher than that expected for the target compound in the TSC (Table 2, first 22 rows), while those for five others (NAT, NAB, NNK, B[a]P, 4-aminobiphenyl) were prepared at a concentration of 100 mg l−1, several orders of magnitude higher than the concentration expected for these analytes (Table 2).
The final solutions for the overspiking experiments were obtained by adding a 10% (v/v) aliquot of a previously prepared standard solution to the TSC. The concentrations of the standard solutions for the overspiking experiments of HPHCs ranged from 20 to 100 mg l−1.
NMR spectral acquisition parameters
Spectra were acquired by a 11.7 T 500 MHz NMR spectrometer (Bruker, Germany) equipped with a 5 mm TCI cryoprobe using the noesypr1d pulse program. All spectra were acquired and processed using Topspin 2.13 patch level 6 (Bruker). The noesypr1d pulse sequence incorporates a low-power saturation of the pre-saturated resonance during the nuclear Overhauser effect mixing time in order to reduce the intensity of the residual exchangeable solvent resonance of deuterated methanol.The following acquisition parameters were used for data collection: 90° pulse length, 7.75 μs; spectral width, 19.9947 ppm; acquisition mode, digital quadrature detection; unrecorded FIDs (Free Induction Decay), 16; recorded FIDs, 512; offset frequency, 4.945 ppm; relaxation delay, 10 seconds; mixing time, 200 ms; and acquisition time, 3.277 seconds. Spectra were acquired at 300 K. These parameters gave a total experimental time of approximately 2 hours.
Statistical treatment of data
Spectroscopy data were subjected to statistical analysis during the validation stage of the study to assess the reproducibility of the method and to facilitate the comparison of NMR spectra obtained from TSC extracts of different cigarette types. NMR data were processed by FELIX software (Accelrys, USA). A sine-bell shaped window function phase-shifted by 90° was applied over all points prior to Fourier transformation, phase and baseline correction. The chemical shifts of all data were referenced to the TSP peak at 0 ppm. The area of this peak was set to unity for all spectra acquired.Statistical analysis was carried out with Metabolab, a custom-written graphical user interface for Matlab version 7.4.0.287 (R2007a) (Mathworks, UK).52
Data were binned using the undecimated wavelet transform to remove noise and to perform peak alignment.53
Results & discussion
As mentioned in the introduction, the concept of non-targeted analysis has been progressing rapidly in a variety of areas including, the medical and food sectors where NMR spectroscopy is one of the principal tools for profiling the composition of foods, beverages and biological fluids such as blood and urine.54 Extensive public databases of NMR spectra have been built containing data from a wide range of metabolites. These are often matrix specific and developed for use in metabolomics/metabonomics studies relating to human, animal or plant metabolites for example. Here a similar approach has been taken in developing an NMR spectral database of the key toxic components of tobacco smoke.Previous studies using NMR spectroscopy within the tobacco sector have generally involved investigating individual compounds and their dynamics, primarily in mainstream smoke/aerosol particulate matter.43,44 Isolated studies such as Barsanti et al.42 have generated useful initial information about the composition of tobacco smoke particulates using NMR spectroscopy. The NMR assignments presented here provide a useful resource to support further studies where previously little information about the NMR chemical shifts in relation to mainstream cigarette smoke was known.
Detection of Hoffmann analytes in 3R4F TSC
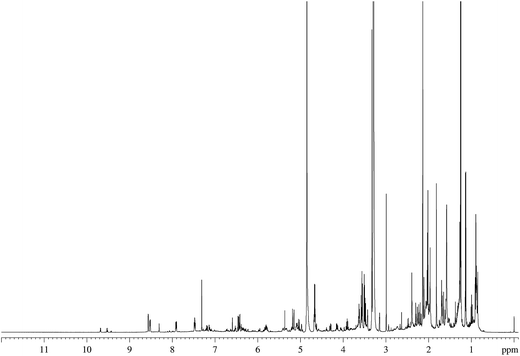
Thirty-three of the original Hoffman analytes were used to assess the feasibility of our analytical approach (Table 2). The main criteria for selecting these analytes were NMR amenability (i.e., the presence of non-exchangeable protons) and their relevance to future FDA regulation.The TSC of 3R4F cigarettes was selected as an initial reference sample to facilitate a comparison between the chemical shifts observed for TSC and those observed for analytical standards. An example of the NMR spectrum of the 3R4F TSC is shown in Fig. 2 (see also ESI Fig. S1†).
Eleven Hoffmann analytes that were expected to be present in TSC at concentrations equivalent to 1 μg per cigarette or lower (Table 2, resorcinol to 4-aminobiphenyl) were evaluated and found to be below the current detection limits of the 1H NMR system. Using standard additions, six of these substances (resorcinol, quinoline, NNN, 1-aminonaphthalene, 2-aminonaphthalene, 3-aminobiphenyl) gave sufficiently intense NMR signals to enable their detection in the TSC.
Standard addition experiments at higher concentrations were conducted for the other 5 compounds (NAT, NAB, NNK, B[a]P and 4-aminobiphenyl) in order to record their characteristic chemical shifts for future reference.
In total, 20 of the 33 target Hoffmann substances were identified in the TSC from the 3R4F cigarette, and their detailed chemical shift data were recorded in deuterated methanol in both the presence and absence of TSC (ESI Table S2†). These data form the basis of an initial database of NMR chemical shifts for toxicants in 3R4F TSC. Thirteen substances (1,3-butadiene, butyraldehyde, m-cresol, resorcinol, quinoline, 1-aminonaphthalene, 2-aminonaphthalene, 3-aminobiphenyl, 4-aminobiphenyl, NAB, NAT, NNK, NNN and B[a]P) were not detected in the mainstream cigarette TSC. Among these, however, only 1,3-butadiene and butyraldehyde were expected to be present above the LOD of the method (estimated as 10 μg per cigarette).
1,3-Butadiene was not detected in TSC, probably because of evaporative losses during sample transport. When it was spiked directly into the TSC at a concentration of 7.84 mg l−1, 1,3-butadiene was detected, allowing its semi-quantification. Butyraldehyde did not display any uniquely identifiable peaks within the NMR spectrum of TSC (ESI Table S2†).
Validation of the NMR protocol for detecting the Hoffmann analytes
Twenty compounds were confirmed during the validation stage: acetaldehyde, isoprene, nicotine, acetone, acrolein, toluene, catechol, hydroquinone, formaldehyde, acrylonitrile, propionaldehyde, crotonaldehyde, butanone, benzene, pyridine, phenol, styrene, o-cresol, m-cresol and p-cresol. Similarly, 13 compounds were present at levels below the limit of detection and therefore not detected directly in 3R4F TSC: 1,3-butadiene, butyraldehyde, resorcinol, quinoline, 1-aminonaphthalene, 2-aminonaphthalene, 3-aminobiphenyl, 4-aminobiphenyl, NAB, NAT, NNK, NNN and B[a]P.The repeatability of the analytical procedure was assessed by analyzing five replicates of 3R4F TSC. The relative standard deviation (RSD, %) of the signals of the NMR spectra was calculated to determine the spread of the results.
The spectral profiles of the replicates were averaged by using the method of adaptive binning with undecimated wavelets.53Fig. 3 shows a stacked plot of the five replicate spectra, along with a plot of the observed RSD. An acceptable RSD of less than 5 was obtained for chemical shifts between 0 and 5.5 ppm, and those between 6 and 10 ppm (Fig. 3 and ESI Fig. S2†).
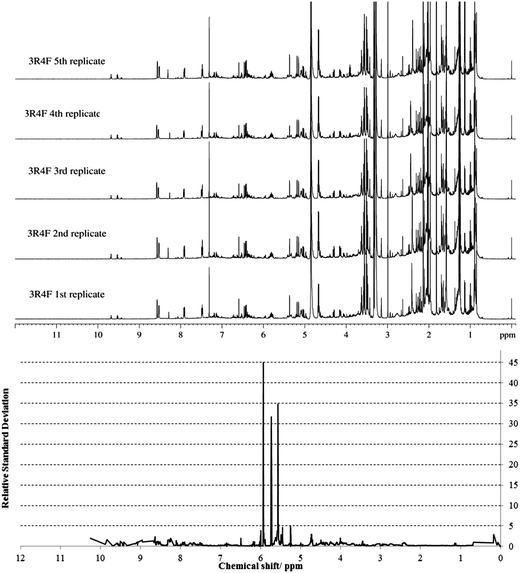 | ||
| Fig. 3 Stacked plot of the NMR spectra of five replicates of 3R4F smoke condensate, along with a plot of the relative standard deviation. | ||
However, higher RSDs were observed for chemical shifts between 5.5 and 6 ppm. This NMR region was characterized by the resonances of 1,3-butadiene, as confirmed by overspiking experiments, the volatility of which might explain the variation observed between replicates.
Nevertheless, the low RSD values obtained in all other NMR regions showed that high experimental reproducibility was achieved. This reproducibility was further confirmed by the consistency of the chemical shifts obtained for the five replicates of 3R4F TSC (ESI Table S3†). The acquisition of consistent replicate data for 3R4F thus confirms the robust nature of the preparation procedure and the stability of the NMR instrumentation. The analytical method established using the 3R4F NMR protocol was then applied to TSC prepared from CM6, which differs in blend composition; mainstream smoke tar and nicotine yield (Table 1). The CM6 TSC chemical shifts of the resonance peaks in each spectrum were compared with those reported in the 3R4F database.
The same 20 Hoffmann analytes detected in 3R4F TSC during development of the protocol were found in CM6 TSC. Similarly, 13 analytes present below the LOD were not detected directly in CM6 TSC. Fig. 4 and ESI Fig. S3† show pairwise comparisons of the NMR spectra of 3R4F and CM6. The results of the protocol validation are reported in detail in ESI Tables S3–S5;† semi-quantitative data for the Hoffmann analytes in 3R4F and CM6 TSC are summarized in Table 3.
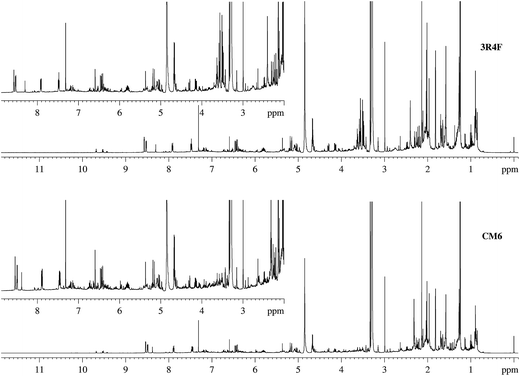 | ||
| Fig. 4 Stacked plot of the NMR spectra of 3R4F and CM6 smoke condensates. The NMR spectral profiles of these two samples showed a difference of 10% when peak areas where compared. | ||
| Hoffmann analyte | Conc. in 3R4F51 (mg per cig) | NMR semi-quantitative data (mg per cig) | |
|---|---|---|---|
| 3R4F | CM6 | ||
| Acetaldehyde | 0.469 | 0.630 | 0.333 |
| Isoprene | 0.347 | 0.373 | 0.273 |
| Nicotine | 0.730 | 0.592 | 0.438 |
| Acetone | 0.199 | 0.220 | 0.128 |
| 1,3-Butadiene | 0.031 | 0.101 | 0.088 |
| Acrolein | 0.052 | 0.042 | 0.020 |
| Toluene | 0.065 | 0.078 | 0.109 |
| Catechol | 0.039 | 0.058 | 0.057 |
| Hydroquinone | 0.031 | 0.026 | 0.025 |
| Formaldehyde | 0.025 | 0.021 | 0.012 |
| Acrylonitrile | 0.011 | 0.009 | 0.006 |
| Propionaldehyde | 0.045 | 0.167 | 0.105 |
| Crotonaldehyde | 0.011 | 0.009 | 0.007 |
| 2-Butanone | 0.052 | 0.076 | 0.045 |
| Benzene | 0.043 | 0.032 | 0.022 |
| Pyridine | 0.005 | 0.018 | 0.017 |
| Styrene | 0.004 | 0.009 | 0.007 |
TSC for NMR was generated as a mainstream smoke extract in deuterated methanol that was directly analyzed by NMR. The NMR and chromatographic yields51 show good agreement with ratios close to 1, thereby demonstrating the potential of NMR as a rapid comprehensive screening technique (Table 3).
Slightly higher ratios were observed in the case of 1,3-butadiene and some carbonyl substances, possibly owing to the very low temperature of the impingers, which was maintained at −70 °C during whole-smoke generation, sample extraction, storage, shipment and NMR analysis in an attempt to minimize evaporative losses.
The 3R4F NMR data were further theoretically evaluated as a complementary technique to enable multi-residue analysis of mainstream tobacco smoke. As indicated above, 20 smoke constituents were directly detected in TSC and 13 analytes require further concentration to be directly detected. Employing the current sample preparation, 1H HR NMR would allow simultaneous measurement of carbonyls, phenols, volatile hydrocarbons and nitrogen heterocycles of the Hoffmann list with only two analytes requiring concentration or modification in sample preparation, resorcinol (10×) and quinoline (50×), Table 4. (It should be noted that quinoline and butyraldehyde belong to Hoffmann analytes but are not on FDA HPHC list.) Thus, 20 out of 24 substances in 4 classes can be measured directly replacing 4 analyses with one.
| Group | Analytes | Concentration required? |
|---|---|---|
| a No isolated NMR signal. Not on FDA FCTC list. b Evaporative losses/handling issues. | ||
| Carbonyls | Methyl ethyl ketone | No |
| Acetaldehyde | No | |
| Acetone | No | |
| Acrolein | No | |
| Butyraldehyde | Seea | |
| Croton aldehyde | No | |
| Formaldehyde | No | |
| Propion aldehyde | No | |
| Phenols | Catechol | No |
| Hydroquinone | No | |
| m + p-Cresol | No | |
| o-Cresol | No | |
| Phenol | No | |
| Resorcinol | 10× | |
| Organics | Acrylonitrile | No |
| Volatile hydrocarbons | 1,3-Butadiene | Seeb |
| Benzene | No | |
| Isoprene | No | |
| Toluene | No | |
| Styrene | No | |
| Nitrogen heterocyclics | Pyridine | No |
| Quinoline | 50× | |
| Nicotine | No | |
The very low abundance genotoxic substances, i.e. B[a]P, NNN and NNK necessities more selective analysis.
NMR detection of a further 34 HPHCs on the FDA list
To expand the database of NMR-detectable toxicants in TSCs, the 3R4F extract was tested for the presence of a further 34 HPHCs from the FDA list.15 Standards of these compounds were fortified into the 3R4F TSC extract and their chemical shifts were recorded by HR 1H NMR. Overspiking of the HPHCs in the TSCs was performed by following the same strategy used to detect the Hoffmann analytes. That is, the concentrations of standards of all of the FDA substances in deuterated methanol were chosen in order to detect an increase in the peak response of about 10%. This process required a heuristic (trial and error) approach based on an assumption about the approximate abundance of the target compounds in TSC, which was not known a priori.In total, 13 compounds were identified directly in the extract and further eight were tentatively identified (Table 5). The assignment of the NMR signals of eight of the target FDA substances (i.e., benzo(a,h)anthracene, 2,5-dimethylanyline, dibenzo(a,l)pyrene, coumarine, acrylamide, benz(a)anthracene, N-nitrosodiethanolamine and benzo(k)fluoranthene) did not clearly confirm the presence of these compounds in the TSC. In other words, when the chemical shifts were recorded, it was concluded that the number of peaks attributed to these eight substances was not sufficient to confirm their presence with reasonable certainty (Table 5).
| # | Compound | Detected in 3R4F TSC |
|---|---|---|
| 1 | Acetamide | Yes |
| 2 | Nitrobenzene | No |
| 3 | Dibenzo(a,h)pyrene | No |
| 4 | Benzo(a,h)anthracene | Uncertain |
| 5 | Caffeic acid | Yes |
| 6 | Dibenzo(a,e)pyrene | No |
| 7 | 2,5-Dimethylanyline | Uncertain |
| 8 | Dibenzo(a,l)pyrene | Uncertain |
| 9 | o-Anisidine | Yes |
| 10 | Chrysene | Yes |
| 11 | 5-Methyl chrysene | Yes |
| 12 | 2,3-Benzofuran | Yes |
| 13 | Pyrrolidine | Yes |
| 14 | N-Methylethylamine | Yes |
| 15 | Coumarine | Uncertain |
| 16 | Acrylamide | Uncertain |
| 17 | 4-(Nitrosomethylamino)-1-(3-pyridyl)-1-butanone | Yes |
| 18 | Benz(a)anthracene | Uncertain |
| 19 | Naphtalene | No |
| 20 | Urethane | No |
| 21 | Indeno(1,2,3-cd)pyrene | No |
| 22 | Anabasine | Yes |
| 23 | Ethylbenzene | Yes |
| 24 | N-Nitrosodiethylamine | No |
| 25 | N-Nitrosodiethanolamine | Uncertain |
| 26 | Benzo(b)fluoranthene | No |
| 27 | Vinylacetate | No |
| 28 | N-Nitrosomorpholine | Yes |
| 29 | o-Toluidine | Yes |
| 30 | Propylene oxide | No |
| 31 | 2-Nitropropane | No |
| 32 | Benzo(k)fluoranthene | Uncertain |
| 33 | N-Nitrosopyrrolidine | No |
| 34 | N-Nitrosopiperidine | No |
The chemical shifts of the compounds that were identified were recorded in detail. In addition to 3R4F TSC prepared from 5 and 10 cigarettes, CM6 TSC was evaluated for the presence of the chemical shifts of these 13 HPHCs in order to monitor sample variation in relation to the type of tobacco blend in the smoke extract (ESI Table S6†).
Conclusion
An optimized 1H-NMR spectroscopy protocol has been developed and validated to identify NMR-amenable Hoffmann analytes and to build a database of chemical shifts in deuterated methanol extracts enabling fast and comprehensive screening of a mainstream tobacco smoke condensate generated from the Kentucky Reference 3R4F cigarette smoked under ISO smoking conditions.Among 33 potentially NMR-amenable toxicants selected from the Hoffmann list, 20 (66%) were identified directly in the 3R4F cigarette TSC by their chemical shifts.
Using some modification of the sample collection and extraction, a further 2–5 Hoffmann analytes (quinoline, resorcinol, NAT, NNN and NNK) are estimated to be detected directly in TSC. In addition, 13 HPHCs out of a further 34 compounds from the FDA list were successfully detected in the 3R4F TSC by using an overspiking technique.
As a part of the validation process, the HR 1H-NMR protocol was successfully used to detect the same toxicants in the TSC of other type of cigarette (CORESTA monitor test piece, CM6). The sample preparation required for NMR is simple, and the total time needed to screen the substances in TSC is approximately 2 hours. The LOD of this method for the selected substances in TSC was estimated to be approximately 10 μg per cigarette.
In summary, the presented results demonstrate the feasibility of HR 1H NMR spectroscopy as a rapid, non-destructive method for assessing a wide range of toxicants in tobacco mainstream smoke and providing comprehensive data packages complimentary to chromatographic methods.
The primary focus of the work presented here was the simultaneous detection of multiple toxic analytes with a focus on establishing a NMR spectroscopy method capable of at least semi-quantification of the analytes, thus reducing the time and effort taken in comparison to the use of established methods.
The method performs acceptably for substances present at μg per cigarette levels in mainstream smoke, but would require significant increase in sensitivity to apply to genotoxic substances that are present at low ng per cigarette levels in mainstream smoke.
Acknowledgements
We thank technical experts at the Food and Environment Research Agency (Sand Hutton, York, YO41 1LZ, UK) for collection and interpretation of NMR data. We also thank Jason Adamson (BAT Research and Development) for designing the Table of Contents image and Fig. 1.Notes and references
- B. J. Ingebrethsen, Recent Adv. Tob. Sci., 1986, 12, 54–142 Search PubMed
.
- M. F. Dube and C. R. Green, Recent Adv. Tob. Sci., 1982, 8, 42–102 Search PubMed
.
-
M. R. Guerin, in Banbury Report No. 3: a Safe Cigarette?, ed. G. B. Gori and F. G. Bock, Cold Spring Harbor Laboratory, Cold Spring Harbor, 1980, pp. 191–204 Search PubMed
.
- M. R. Guerin, C. E. Higgins and R. A. Jenkins, Atmos. Environ., 1987, 21, 291–297 CrossRef CAS
.
- G. Löfroth, Mutat. Res., 1989, 222, 73–80 Search PubMed
.
- C. R. Green and A. Rodgman, Recent Adv. Tob. Sci., 1996, 22, 131–304 Search PubMed
.
- D. Hoffmann and I. Hoffmann, Beitr. Tabaksforsch. Int., 1998, 18, 49–52 Search PubMed
.
- A. Thielen, H. Klus and L. Muller, Exp. Toxicol. Pathol., 2008, 60, 141–156 CrossRef CAS PubMed
.
- M. Borgerding and H. Klus, Exp. Toxicol. Pathol., 2005, 57, 43–73 CrossRef CAS PubMed
.
- D. L. Roberts, Recent Adv. Tob. Sci., 1988, 14, 49–81 Search PubMed
.
-
R. R. Baker, in Tobacco Production, Chemistry and Technology, ed. D. L. Davis and M. T. Nielson, Blackwell Science, Oxford, 1999, pp. 398–439 Search PubMed
.
-
H. Wakeham, in The Chemistry of Tobacco and Tobacco Smoke, ed. I. Schmeltz, Plenum Press, New York, 1972, pp. 1–20 Search PubMed
.
-
A. Rodgman and T. A. Perfetti, The Chemical Components of Tobacco and Tobacco Smoke, CRC Press, Boca Raton, 2nd edn, 2013 Search PubMed
.
-
Food and Drug Administration, Overview of the Family Smoking Prevention and Tobacco Control Act, updated June 2015, http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM336940.pdf, accessed June 2015 Search PubMed
.
-
Food and Drug Administration, Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List, April 2012, http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf, accessed February 2015 Search PubMed
.
-
Food and Drug Administration, Guidance for Industry: Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act, March 2012, http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297828.pdf, accessed June 2015 Search PubMed
.
- S. M. Charles, C. Jia, S. Batterman and C. Goodwin, Environ. Sci. Technol., 2008, 42, 1324–1331 CrossRef CAS PubMed
.
- S. M. Charles, S. A. Batterman and C. Jia, Atmos. Environ., 2007, 41, 5371–5384 CrossRef CAS
.
- Q. J. Ye, J. Chromatogr. A, 2008, 1213, 239–244 CrossRef CAS PubMed
.
- G. M. Polzin, E. E. Kosa-Maineas, D. L. Ashley and C. H. Watson, Environ. Sci. Technol., 2007, 41, 1297–1302 CrossRef CAS PubMed
.
- J. L. Li, Y. L. Feng, C. J. Xie, J. Huang, J. Z. Yu, J. L. Feng, G. Y. Sheng, J. M. Fu and M. H. Wu, Anal. Chim. Acta, 2009, 635, 84–93 CrossRef CAS PubMed
.
- X. Pang and A. C. Lewis, Sci. Total Environ., 2011, 409, 5000–5009 CrossRef CAS PubMed
.
- Y. S. Ding, X. J. Yan, R. B. Jain, E. Lopp, A. Tavakoli, G. M. Polzin, S. B. Stanfill, D. L. Ashley and C. H. Watson, Environ. Sci. Technol., 2006, 40, 1133–1138 CrossRef CAS PubMed
.
- N. P. Kulshreshtha and S. C. Moldoveanu, J. Chromatogr. A, 2003, 985, 303–312 CrossRef CAS PubMed
.
- S. C. Moldoveanu and M. J. Kiser, J. Chromatogr. A, 2007, 1141, 90–97 CrossRef CAS PubMed
.
- J. Cai, B. Liu and Q. J. Su, J. Chromatogr. A, 2003, 1017, 187–193 CrossRef CAS PubMed
.
- M. Brokl, L. Bishop, C. G. Wright, C. Liu, K. McAdam and J. F. J. Focant, Sep. Sci., 2013, 36, 1037–1044 CrossRef CAS PubMed
.
- E. Kupče, R. Freeman, G. Wider and K. J. Wuthrich, J. Magn. Reson., Ser. A, 1996, 122, 81–84 CrossRef
.
- H. Kessler, H. Oschkinat, G. Griesinger and W. J. Bermel, Magn. Reson., 1986, 70, 106–133 CAS
.
- R. Freeman, Chem. Rev., 1991, 91, 1397–1412 CrossRef CAS
.
- C. Pontoizeau, H. Torsten, P. Toulhoat, B. Elena-Herrmann and L. Emsley, Magn. Reson. Chem., 2010, 48, 727–733 CrossRef CAS PubMed
.
- P. Krishnan, N. J. Kruger and R. G. Ratcliffe, J. Exp. Bot., 2004, 56, 255–265 CrossRef PubMed
.
- L. A. Pieters and A. J. Vlietinck, J. Pharm. Biomed. Anal., 1989, 7, 1405–1417 CrossRef CAS PubMed
.
- B. Meyer, T. Weimar and T. Peters, Eur. J. Biochem., 1997, 246, 705–709 CAS
.
- L. Mannina, A. P. Sobolev and A. Segre, Spectrosc. Eur., 2003, 15, 6–14 CAS
.
- A. J. Charlton, J. D. Donarski, S. A. Jones, D. B. May and K. C. Thompson, J. Environ. Monit., 2006, 8, 1106–1110 RSC
.
- A. J. Charlton, P. Robb, J. A. Donarski and J. Godward, Anal. Chim. Acta, 2008, 618, 196–203 CrossRef CAS PubMed
.
- D. W. Lachenmeier, T. A. Breaux, T. Kuballa, C. Schlee and Y. B. Monakhova, Food Chem., 2014, 159, 230–235 CrossRef CAS PubMed
.
- Y. B. Monakhova, T. Kuballa and D. W. Lachenmeier, Appl. Magn. Reson., 2012, 42, 343–352, DOI:10.1007/s00723-011-0309-2
.
- D. W. Lachenmeier, E. Humpfer, F. Fang, B. Schutz, P. Dvortsak, C. Sproll and M. Spraul, J. Agric. Food Chem., 2009, 57, 7194–7199 CrossRef CAS PubMed
.
- J. Hahn, Y. B. Monakhova, J. Hengen, M. Kohl-Himmelseher, J. Schussler, H. Hahn, T. Kuballa and D. W. Lachenmeier, Tob. Induced Dis., 2014, 12, 23, DOI:10.1186/s12971-014-0023-6
.
- K. C. Barsanti, W. Luo, L. M. Isabelle, J. F. Pankow and D. H., Magn. Reson. Chem., 2007, 45, 167–170 CrossRef CAS PubMed
.
- J. Pankow, A. D. Tavakoli, W. Luo and L. M. Isabelle, Chem. Res. Toxicol., 2003, 16, 1014–1018 CrossRef CAS PubMed
.
- J. Pankow, K. C. Barsanti and D. H. Peyton, Chem. Res. Toxicol., 2003, 16, 23–27 CrossRef CAS PubMed
.
-
ISO, Cigarettes–Determination of Total and Nicotine-free Dry Particulate Matter Using a Routine Analytical Smoking Machine, ISO 4387, Geneva, 2000 Search PubMed
.
-
ISO, Methods for Chemical Analysis of Tobacco and Tobacco Products Ð Part 12: Determination of Nicotine in Smoke Condensate of Cigarettes (Gas-chromatographic Method), ISO 10315, Geneva, 2000 Search PubMed
.
-
ISO, Cigarettes–Determination of Carbon Monoxide in the Vapour Phase of Cigarette Smoke–NDIR Method, ISO 8454, ISO, Geneva, 2007 Search PubMed
.
-
ISO, Routine Analytical Cigarette Smoking Machine—definition and Standard Conditions, ISO 3308, ISO, Geneva, 2000 Search PubMed
.
-
ISO, Tobacco and Tobacco Products–atmosphere for Conditioning and Testing, ISO 3402, ISO, Geneva, 1999 Search PubMed
.
-
J. Adamson, L. Haswell, G. Phillips and M. D. Gaca, in Bronchitis, ed. I. Martin-Loeches, InTech, Rijeka, 2011 Search PubMed
.
- A. Eldridge, T. R. Betson, M. Vinicius Gamma and K. McAdam, Regul. Toxicol. Pharmacol., 2015, 71, 409–427 CrossRef CAS PubMed
.
- C. Ludwig and U. L. Gunther, BMC Bioinf., 2011, 12, 366 CrossRef CAS PubMed
, http://ww.biomedcentral.com/1471-2105/12/366.
- R. A. Davis, A. J. Charlton, J. Godward, S. A. Jones, M. Harrison and J. C. Wilson, Chemom. Intell. Lab. Syst., 2007, 85, 144–154 CrossRef CAS
.
- J. S. McKenzie, J. A. Donarski, J. C. Wilson and A. J. Charlton, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 44(4), 336–359 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ay00849f |
| This journal is © The Royal Society of Chemistry 2016 |