Detection of signature forgery with erasable pens using paper spray mass spectrometry (PS-MS)
Marina
Jurisch
and
Rodinei
Augusti
*
Departamento de Química, Instituto de Ciências Exatas, Universidade Federal de Minas Gerais, Belo Horizonte 31270-901, Brazil. E-mail: augusti.rodinei@gmail.com; Tel: +55 31 3409 5734
First published on 26th April 2016
Abstract
Paper spray mass spectrometry (PS-MS) is applied to a fast, sensitive and reproducible detection of forgery in signatures made with erasable pens that use a chemical method to remove inks (other than the usual physical methods). A forgery process was simulated by signing over a previously erased signature and triangular sections were cut out of the paper in order to perform the PS-MS analysis. No sample preparation was required as the analysis was conducted directly on the triangular paper by simply wetting it with methanol and applying high voltage. Analysis of the original, erased and forged signatures was made following the same procedure. The results show that the original and the forged signatures were promptly distinguished by their mass spectrometry profiles (fingerprints). Hence, the appearance of diagnostic ions (of m/z 172 and m/z 321) in the mass spectrum of the counterfeit signature can be used to quickly and reliably characterize this novel and potentially harmful type of forgery.
Introduction
In forensic science, many fields have growing needs for cheap, fast and reliable methods of chemical analysis. In order to attend to the constant flow of evidence coming into the laboratories, rapid and precise methods are being developed. The investigation of suspect documents, e.g. that may have the date, number or signature altered, is of great importance in forensic laboratories.1 Analysis of such documents usually starts with visual and physical methods, which are of low cost and require close to no special equipment (microscopes and different light sources, for instance).2 Usually, however, visual and physical methods need to be complemented by chemical analysis to determine whether entries have been modified or added post factum.3All writing inks are made, primarily, of a vehicle and particular coloured components. Chemical analysis aims to differentiate entries based on specific properties of such compounds.3 Erasable pens have been commercially available since the late 1970s and made the identification of forgeries more difficult. These types of inks are usually erasable by physical methods, i.e. friction or changes in temperature.
Forensic analysis of ink in documents is commonly made by using complex, time-consuming, and laborious techniques including high performance liquid chromatography (HPLC), gas chromatography (GC), thin-layer chromatography (TLC), capillary electrophoresis (CE), Fourier transform infrared spectroscopy (FTIR), and microspectrophotometry, among others.1–4
Ambient ionization methods in mass spectrometry have been gaining a significant amount of interest since 2004, with the introduction of desorption electrospray ionization (DESI).5 Much of this is due to the various advantageous aspects these methods present, which include close to no sample preparation (a time-consuming step) and the possibility of direct analysis of samples, excluding the need for an extraction step.5
Paper spray (PS) is a versatile ambient ionization method first proposed by Cooks and co-workers in 2010.6 Since then it has been vastly used in many different fields such as quantification of drugs of abuse7,8 and of therapeutic use9 in blood, analysis of biological tissues10 and microorganisms,11 and analysis in foodstuff,12–15 among other applications. The many uses of paper spray mass spectrometry (PS-MS) revolve around its many remarkable qualities, which include its rapidness, cheapness and simple procedure.
The mechanism employed by the PS technique is quite simple. A paper – chromatographic, filter or even plain white – is cut out into a triangular shape, in order to have a macroscopic sharp point, and the triangular paper is placed directly in front of the mass spectrometer inlet. The analyte (solid or liquid) is directly placed on the paper, which is then wetted with a solvent, usually methanol or a solution of methanol and deionized water. High voltage is applied to the wet paper generating a spray similar to that produced by electrospray ionization (ESI).6,16
A new set of pens that use a chemical method to erase writing inks was recently released.17 It is reasonable to assume that these pens can be employed in a potentially damaging action: forgery in written documents. Hence, the present manuscript demonstrates for the first time that paper spray mass spectrometry (PS-MS) can be efficiently used as an alternative method to detect this new type of fraud.
Experimental
Inks and signatures
Pens of the brand Stabilo®, of erasable and non-erasable qualities, were purchased from local stores. Since the chemical components of such pens are kept secret by the company, the authors of the present manuscript have no wish to unveil their structures. The following pens were utilized herein: eraser white (color code: 00), erasable blue (color code: 0041) and non-erasable blue (color code: 41). Each code refers to the erasable quality of each pen. A line of each individual pen was drawn onto pieces of paper of triangular shape (approximately 10 mm height and 5 mm base width), which were cut out from an ordinary white office paper sheet (Ripax Laser 75 g m−2). The chemical profile of each pen was then recorded by PS-MS analysis, as well as a blank spectrum of the paper with no writings. To simulate a real forgery situation, a proof-of-principle experiment was designed, in which a volunteer signed six times with the erasable blue pen in a white office paper sheet (Fig. 1, the experiments were performed in duplicate). Four of these signatures were erased with the eraser white pen whereas two of them were maintained unaltered (Fig. 1A/D). In sequence, while two of the erased portions (Fig. 1B/E) were left intact, a second volunteer signed over the two remaining erased areas (Fig. 1C/F). Triangular sections (approximately 10 mm height and 5 mm base width) representative of each area (original signatures, erased portions and forged signatures) were cut off, as indicated in Fig. 1, and directly analyzed by PS-MS.For comparison purposes, easy ambient sonic-spray ionization (EASI) experiments were conducted following the same forgery steps. The experiments were carried out using a Q Extractive Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA, USA) working in the positive-ion mode and recording data within the 100–1000 m/z range. Additional details on other experimental conditions and features of this technique are provided elsewhere.1,18
Paper spray and instrumentation
The PS-MS system utilized in the experiments is shown in Fig. 2. It consisted of a mobile platform with three-dimensional movement where the triangular paper was fixed with a copper clip. The distance between the tip of the paper and the mass spectrometer inlet was set to 10 mm. The paper was wetted with 10 μL of HPLC grade methanol and a high voltage (3 kV) was applied through the metal clip. To prevent any memory effect, a blank paper (with no sample) was used between analyses. All experiments were performed using a Thermo LCQ Fleet mass spectrometer (Thermo Scientific, San Jose, CA, USA) operating in the positive ion mode. The following instrumental parameters were kept constant during the analysis: spray voltage 3.0 kV; capillary voltage 35 V; capillary temperature 275 °C; tube lens voltage 65 V. Full scan mass spectra data were acquired within the 100–1200 mass-to-charge ratio (m/z) range for all the samples analyzed.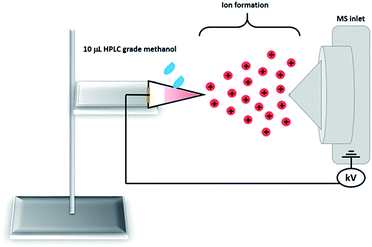 | ||
| Fig. 2 Schematic representation of the PS-MS system used to monitor the forgery process with the erasable/eraser/non-erasable pens. | ||
Results and discussion
All analysis was carried out in the positive ion mode given the lack of the signal response in the negative ion mode. With that, one can infer that the chemical components of the analyzed pens either have basic sites on their structures (that are able to accept protons) or are permanently cationic.In order to achieve the mass spectra with the best quality possible, i.e. with intense, stable and reproducible signals with minimal background noise, the spray voltage (3 kV) and the distance between the paper tip and MS inlet (10 mm) were optimized. The results of the ink analysis of the white eraser, erasable blue and non-erasable blue pens are shown in Fig. 3 (A, B, and C, respectively). The PS(+)-MS spectrum collected for the office paper (blank mass spectrum, Fig. 3) shows intense ions of m/z 413 and 803, both of them being undetectable in the PS(+)-MS spectrum of the simulated forgery experiments (Fig. 4 and 5).
 | ||
| Fig. 3 PS(+)-MS spectrum obtained for the white office paper wetted with methanol and with no writings (blank analysis). | ||
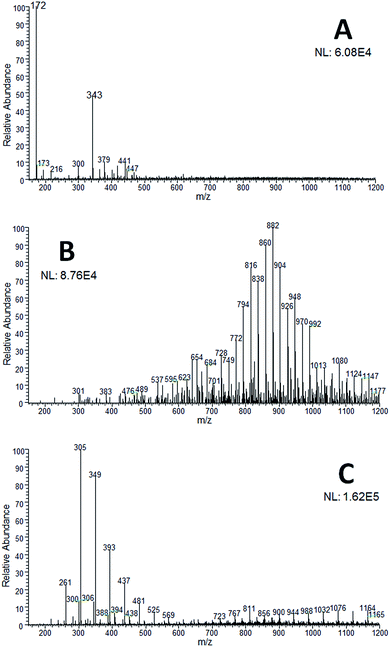 | ||
| Fig. 4 PS(+)-MS spectrum recorded for the inks from (A) the white eraser pen; (B) the erasable blue pen; (C) the non-erasable blue pen. | ||
 | ||
| Fig. 5 PS(+)-MS spectrum obtained for the forgery experiment: (A) erased region (see Fig. 1B/E for the optical image); (B) forged signature (see Fig. 1C/F for the optical image). Note the highlighted ions of m/z 172 and 321 (B) that diagnose adulteration. | ||
The results from the PS(+)-MS analysis demonstrate that the PS(+)-MS spectrum of the white eraser pen (Fig. 4A) has mainly two characteristic ions (of m/z 172 and 343) that are not detectable in neither of the PS(+)-MS spectra of the erasable and non-erasable blue pens (Fig. 4B and C, respectively). In contrast, the erasable (Fig. 4B) and non-erasable (Fig. 4C) blue pens display characteristic mass spectra of polymers. Although the profiles are quite different (comparing Fig. 4B and C), both mass spectra present ions with the same difference between adjacent signals (of 44 Da), which, most likely, refers to a CH2CH2O group. Both mass spectra also have two different sets of polymeric chains. For the erasable blue pen (Fig. 4B) they merge together in the 700–1200 m/z range, but for the non-erasable blue pen (Fig. 4C) they appear as two distinct clusters: of m/z 200–600 (mainly) and m/z of 700–1200. Thus, these results clearly indicate that it is quite simple to differentiate between each pen as their mass spectrometry fingerprints are quite distinctive.
In the experiments that simulated signature forgery, the white eraser pen took just under 40 s to completely erase the original signatures made with the erasable blue pen (see Fig. 1A/D and B/E). After 20 min the surface was completely dry and the non-erasable blue pen was used to write on top of it (Fig. 1C/F). Note that a simple visual inspection of the final signatures (Fig. 1C/F) could not reveal any sign of forgery, which demonstrates the high potential damage that this illicit practice can cause. Hence, such “suspect documents” were subjected to chemical analysis via PS-MS to confirm or discard the forgery suspicions. The resulting PS(+)-MS spectrum (Fig. 5A) of the erased region (Fig. 1B/E) shows that the polymeric clusters of the erasable blue pen (Fig. 4B) were either suppressed by the ions of the eraser white pen (most likely) or reacted forming by-products, this last assumption being supported by the appearance of new ions of m/z 278, 321, 419, which are different from the typical ions of the white eraser pen (of m/z 172 and 343, Fig. 4A).
Based on the data obtained for the forged signature (Fig. 1C/F), it is found that the PS(+)-MS spectrum (Fig. 5B) is compatible with that of the non-erasable blue pen (Fig. 4C) except for the presence of two noticeable ions of m/z 172 (diagnostic for the white pen, Fig. 4A) and 321, which is assumed to arise from a reaction between the ingredients of the two pens, i.e. white and erasable blue. Such two ions do not appear prior to the forgery in the PS(+)-MS spectrum of the non-erasable blue pen (Fig. 4C) and can therefore be used as irrefragable evidence that the original signature, made with an erasable pen, was erased and a new signature was written to replace it.
MS/MS experiments were carried out but even at high collision energies (approximately 30 manufacturer's units) no fragments were observed for the ions of m/z 172, 278, 321, 343 and 419 suggesting that they possess stable structures that are quite resistant towards collision-induced dissociation (CID).
Another proof-of-point experiment was performed using easy ambient sonic-spray ionization (EASI) in order to verify whether this type of forgery could be detected by a well-established ambient ionization method for the analysis of forgeries in documents.1,18 As displayed in Fig. 6, the results are similar to the ones obtained for the forged document (Fig. 1C/F) using the PS-MS technique (Fig. 5B). Hence, the mass spectrum shows both the diagnostic ions for adulteration, of m/z 172 and 321, as well as the same pattern verified for the non-erasable blue pen used to make forgery.
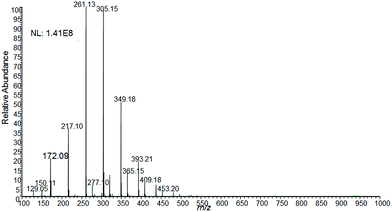 | ||
| Fig. 6 EASI(+)-MS spectrum of the forged signature (see Fig. 1C/F for the optical image). The ions proposed to be diagnostic for the adulteration (m/z 172 and 321) are detectable in this mass spectrum. The same pattern observed for the non-erasable blue pen (Fig. 4C) is also noticeable. | ||
Conclusions
The application of paper spray mass spectrometry made possible a prompt detection of a forged signature made using a new set of pens that basically employ a chemical method to remove inks. The PS-MS system is shown to be efficient providing intense, stable and reproducible signals with a good signal-to-noise ratio. Compared to the traditional methods normally used in chemical analysis of inks, PS-MS ionization is less expensive, laborious and time-consuming achieving the same high quality results with even more sensitivity. In comparison with one of the most traditional ambient ionization techniques, e.g. EASI, PS-MS showed a similar performance to diagnose this new and potentially risky type of forgery.5 However, although both techniques were able to detect this type of forgery, PS-MS uses a less complex apparatus that requires little-to-no expertise from the operator. Since PS-MS makes an overall scrutiny of the chemical constituents of a given sample, unquestionable forgery recognition can therefore be promptly achieved. Finally, studies are underway in our laboratory to verify whether different types of paper and degradation (natural and/or accelerated by light) should be pursued to truly establish this as an alternative technique to determine this type of forgery in written documents.Acknowledgements
The authors would greatly like to acknowledge the financial support provided by CNPq (Proc. 456603/2014-0) and FAPEMIG to this research. The authors are also indebted to Professor Marcos Eberlin and Pedro Henrique Vendramini at the ThoMSon Mass Spectrometry Laboratory for their assistance with the EASI experiments.References
- P. M. Lalli, G. B. Sanvido, J. S. Garcia, R. Haddad, R. G. Cosso, D. R. J. Maia, J. J. Zacca, A. O. Maldaner and M. N. Eberlin, Analyst, 2010, 135, 745–750 RSC.
- P. D. Ferreira, D. F. D. E. Silva, R. Augusti and E. Piccin, Analyst, 2015, 140, 811–819 RSC.
- M. R. Williams, C. Moody, L. A. Arceneaux, C. Rinke, K. White and M. E. Sigman, Forensic Sci. Int., 2009, 191, 97–103 CrossRef CAS PubMed.
- D. R. Ifa, L. M. Gumaelius, L. S. Eberlin, N. E. Manicke and R. G. Cooks, Analyst, 2007, 132, 461–467 RSC.
- C. W. Klampfl and M. Himmelsbach, Anal. Chim. Acta, 2015, 890, 44–59 CrossRef CAS PubMed.
- H. Wang, J. J. Liu, R. G. Cooks and Z. Ouyang, Angew. Chem., Int. Ed., 2010, 49, 877–880 CrossRef CAS PubMed.
- R. D. Espy, S. F. Teunissen, N. E. Manicke, Y. Ren, Z. Ouyang, A. van Asten and R. G. Cooks, Anal. Chem., 2014, 86, 7712–7718 CrossRef CAS PubMed.
- Y. Su, H. Wang, J. J. Liu, P. Wei, R. G. Cooks and Z. Ouyang, Analyst, 2013, 138, 4443–4447 RSC.
- N. E. Manicke, P. Abu-Rabie, N. Spooner, Z. Ouyang and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2011, 22, 1501–1507 CrossRef CAS PubMed.
- H. Wang, N. E. Manicke, Q. A. Yang, L. X. Zheng, R. Y. Shi, R. G. Cooks and O. Y. Zheng, Anal. Chem., 2011, 83, 1197–1201 CrossRef CAS PubMed.
- A. M. Hamid, P. Wei, A. K. Jarmusch, V. Pirro and R. G. Cooks, Int. J. Mass Spectrom., 2015, 378, 288–293 CrossRef CAS.
- J. W. Deng and Y. Y. Yang, Anal. Chim. Acta, 2013, 785, 82–90 CrossRef CAS PubMed.
- A. Y. Li, P. Wei, H. C. Hsu and R. G. Cooks, Analyst, 2013, 138, 4624–4630 RSC.
- F. Mazzotti, L. Di Donna, D. Taverna, M. Nardi, D. Aiello, A. Napoli and G. Sindona, Int. J. Mass Spectrom., 2013, 352, 87–91 CrossRef CAS.
- D. Taverna, L. Di Donna, F. Mazzotti, B. Policicchio and G. Sindona, J. Mass Spectrom., 2013, 48, 544–547 CrossRef CAS PubMed.
- R. D. Espy, A. R. Muliadi, Z. Ouyang and R. G. Cooks, Int. J. Mass Spectrom., 2012, 325, 167–171 CrossRef.
- Y. Y. Kao, S. C. Cheng, C. N. Cheng, J. Shiea and H. O. Ho, J. Mass Spectrom., 2014, 49, 445–451 CrossRef CAS PubMed.
- R. Sparrapan, M. N. Eberlin and R. Haddad, Rapid Commun. Mass Spectrom., 2006, 20, 2901–2905 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |

