Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimization of the procedure for efficient dispersion of titanium dioxide nanoparticles in aqueous samples
Janja
Vidmar
ad,
Radmila
Milačič
ad,
Viviana
Golja
bd,
Saša
Novak
cd and
Janez
Ščančar
*ad
aDepartment of Environmental Sciences, Jožef Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia. E-mail: janez.scancar@ijs.si; Tel: +386 1 4773846
bNational Institute of Public Health, Trubarjeva 2, 1000 Ljubljana, Slovenia
cDepartment for Nanostructured Materials, Jožef Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia
dJožef Stefan International Postgraduate School, Jamova 39, 1000 Ljubljana, Slovenia
First published on 7th January 2016
Abstract
The widespread use of titanium dioxide nanoparticles (TiO2NPs) in consumer products has led to an increase of their concentrations in the environment. For reliable determination of their total concentrations, the microwave assisted digestion procedure for the decomposition of nanoscale anatase and rutile was optimized and Ti concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS). To determine the TiO2NP concentration in environmental water samples, sample treatments, which maintain NPs dispersed and stabilized in solution, enabling quantitative transfer of TiO2NPs during the analytical procedure, are of crucial importance. In the present work, several dispersion approaches by the use of different mechanical and ultrasonication procedures in combination with various dispersing agents were examined in order to prepare aqueous suspensions of stable and homogeneously dispersed TiO2NPs. Experiments were performed with commercially available rutile and anatase NPs in MilliQ water. The efficiency of NP dispersion was evaluated by measuring the zeta potential and through the determination of the Ti concentration by ICP-MS after microwave assisted digestion of samples. Among different dispersion approaches, ultrasonication or ultrasonication in combination with dispersing agents, such as polyethyleneimine (PEI 600), ammonium polymethacrylate (Darvan C) or Triton X100, was found to be most effective for dispersing and stabilizing nanoscale rutile in MilliQ water. In order to verify the applicability of ultrasonication and its combination with dispersing agents, river and wastewater samples were spiked with nanoscale anatase and rutile and the stability of TiO2NP dispersions was examined by measuring the zeta potential. The results demonstrated that different environmental conditions, such as the presence of natural organic matter and ionic strength, have a significant influence on the efficiency of dispersion and the stability of TiO2NPs.
1. Introduction
Over the past years, the production of titanium dioxide nanoparticles (TiO2NPs) has been largely increased.1 TiO2NPs are generally synthesized in different crystalline phases (e.g. anatase and rutile) which possess unique properties. The anatase phase is used in antimicrobial applications for air and water purification and in sunscreens due to the highest photocatalytic activity, while rutile is used as a common white pigment additive in cosmetics, pharmaceuticals, paintings and food.2 After their use, TiO2NPs can be discharged into the sewage system and are subsequently released into surface waters, where they can interact with living organisms. A number of studies have been published concerning the potential ecotoxicological risks of TiO2NPs to aquatic organisms, such as green algae,3Daphnia magna,4,5Artemia salina6 and marine phytoplankton.7 Their toxic effects especially towards algae, higher plants, aquatic and terrestrial invertebrates and freshwater fish2 induce gill injury, oxidative stress, and other physiological effects8 or even enhance the uptake and toxicity of other pollutants.9 Their toxic effects in biological systems largely depend on the concentration, surface properties and size,10 as well as on the crystallinity of TiO2NPs.11,12Once NPs enter the environment they can undergo different transformations. Their fate and behaviour depend on physicochemical properties, especially the NP particle size13 as well as the environmental conditions, such as pH, ionic strength (presence of multivalent cations), and their interactions with natural organic matter (NOM) or natural colloids. This leads to NP aggregation, sedimentation, dissolution, chemical transformation and their attachment to natural colloids.14
In order to predict the possible toxic effects of TiO2NPs on living organisms, their concentrations in environmental water samples have to be quantitatively determined. The total Ti concentration in nanoscale TiO2 either suspended in aqueous solutions or in solid matrices has been in general determined after different digestion procedures. TiO2 samples were decomposed by hot-plate acid digestion with HNO3,15,16 (NH4)2SO4/H2SO4, HF/H2SO4, H3PO4,17 microwave assisted digestion with HCl/HNO3/HF17 or HF/HNO318 and alkaline fusion with ammonium persulfate,19 KHSO4, K2S2O7 or Na2CO3.20,21 The determination of total Ti concentrations in digested samples was performed by inductively coupled plasma-optical emission or mass spectrometry (ICP-OES, ICP-MS). The drawback of these procedures was incomplete decomposition of TiO2NPs, resulting in poor recoveries, except in the case of the fusion method with ammonium persulfate where the reported recoveries were higher than 95%.19
Analytical methods for quantification and characterization of NPs (e.g. different types of liquid chromatography (LC) or field flow fractionation (FFF)22–24) require their stabilization in dispersion prior to quantification. These techniques are combined with detection methods, such as dynamic light scattering (DLS),25 nanoparticle tracking analysis (NTA)26 or ICP-MS. The latter one is most suitable for detecting low concentrations of NPs in environmental water samples (<ng mL−1). Recently, a single particle (SP)-ICP-MS approach for quantification and characterization of metal-containing NPs has also been developed.27
To avoid underestimation of NP concentrations in environmental waters, the stability of NPs in dispersion throughout the analytical procedure needs to be ensured. In order to prevent agglomeration and settling of TiO2NPs, various sample preparation techniques were used. The procedure based on quantitative phase transfer of hydrophobically modified TiO2NPs from aqueous samples to an organic solvent was reported.28 The stabilization of TiO2NPs in solution was achieved by varying the pH, concentration of humic acids and alginate29 and magnetic stirring or ultrasonication under different conditions in solution (pH, ionic strength, and organic colloids).25 Moreover, for efficient dispersion of TiO2NPs in aqueous samples different dispersing agents were tested30 considering also the zeta potential as a physico-chemical parameter that influences ion adsorption and electrostatic interactions between charged TiO2NPs.31 In the above sample preparation techniques no attempt was made to check, whether the dispersed TiO2NPs enabled their quantitative determination.
Therefore, the aim of our work was to investigate the conditions, which would ensure the stability of TiO2NPs throughout the analytical procedures for accurate quantification of NPs. Various approaches for stabilization of TiO2NPs in solution (mechanical shaking, ultrasonication, and the use of different dispersing agents) were applied and their efficiencies were verified by the determination of total Ti concentrations in dispersions after microwave assisted digestion by the use of ICP-MS. In addition, the zeta potential was also measured in TiO2NP dispersions. The influence of the sample matrix on the dispersion efficiency was examined in spiked river and wastewater samples.
2. Materials and methods
2.1. Instrumentation
Total Ti concentrations after microwave assisted digestion were determined on an Agilent 7700x ICP-MS (Agilent Technologies, Japan) equipped with a quadrupole mass analyser. Experimental working conditions for ICP-MS (summarized in Table 1) were optimized for plasma robustness and adequate sensitivity.| Parameter | Type/value |
|---|---|
| Sample introduction | |
| Nebulizer | Teflon MiraMist |
| Spray chamber | Scott |
| Skimmer and sampler cone | Ni |
| Sample depth | 7.5 mm |
| Nebulizer pump | 0.1 rps |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Plasma conditions | |
| Forward power | 1550 W |
| Plasma gas flow | 15.0 L min−1 |
| Carrier gas flow (Ar) | 0.98 L min−1 |
| Dilution gas flow (Ar) | 0.3 L min−1 |
| Collision gas flow (He) | 4.3 mL min−1 |
| Auxiliary gas flow | 0.9 L min−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Data acquisition parameters | |
| Data acquisition mode | Spectrum |
| Integration time per isotope | 500 ms |
| Total acquisition time | 17 s |
| Isotopes monitored | 48Ti, 49Ti |
| Isotopes of internal standards | 103Rh |
For stabilization of TiO2NPs during the analytical procedure, different modes of shaking and ultrasonication were applied. Samples were shaken on an orbital shaker Vibromix 40 (Tehtnica, Slovenia) at 300 rpm and a vortex Vibromix 10 (Tehtnica). Ultrasonication was performed using an ultrasonic bath Clifton, SW3H type, Nickel-Electro Ltd. (Weston-super-Mare, United Kingdom), operating at a frequency of 37 kHz, and an ultrasonic homogenizer, 4710 series (Cole-Parmer Instrument Co., USA) equipped with a 6 mm diameter microprobe. It was operated at 10% power output at a frequency of 20 kHz and 50% pulsed operation mode for 3 minutes. Samples were kept on an ice bath during sonication.
A CEM MARS 5 (CEM Corporation, USA) Microwave Acceleration Reaction System equipped with temperature and pressure feedback controls operating at 1600 W was used for digestion of samples.
The zeta potentials of TiO2NPs in MilliQ water, environmental water samples or aqueous solutions with different dispersing agents were measured as a function of pH by using a ZETA PALS Zeta Potential Analyzer (Brookhaven Instruments Corporation, USA). The pH was adjusted by HCl or NaOH and measured by using a pH meter (SevenMulti, Mettler Toledo, Switzerland).
A Mettler AE 163 (Mettler Toledo) analytical balance was used for all weighing.
2.2. Chemicals and materials
MilliQ water (18.2 MΩ cm) (Merck Millipore, USA) was used for sample preparations and sample dilutions.Standard TiO2NP dispersions were prepared using anatase TiO2 nanopowder (<25 nm particle size, spherical shape) (#637254) and rutile TiO2 nanopowder (<100 nm particle size, rod shape with a diameter of about 10 nm and a length of 40 nm) (#637262) (Sigma-Aldrich, USA). The declared specific surface area of anatase and rutile was 45–55 m2 g−1 and 130–190 m2 g−1, respectively. The declared density at 25 °C for anatase and rutile was 3.9 g mL−1 and 4.17 g mL−1, respectively. According to the manufacturer, rutile nanopowder may contain up to 5 wt% silicon dioxide (SiO2) as a surface coating.
For the determination of total Ti concentrations by ICP-MS, dissolved Ti standard solutions (Merck Millipore) of 1000 ± 4 mg L−1 were prepared in 0.1% nitric acid and used for preparation of the calibration curves, while Rh standard solution (25 μg L−1) prepared from the stock standard (Merck) of 1000 ± 10 mg L− 1 was used as an internal standard.
Nitric acid (65% HNO3), hydrochloric acid (30% HCl), hydrofluoric acid (40% HF), boric acid (H3BO3) and ammonium peroxodisulfate ((NH4)2S2O8) supplied from Merck Millipore were used for the microwave assisted digestion of the TiO2NP samples.
In order to test the stability of TiO2NP suspension, different dispersing agents were used: PEI 600 (polyethyleneimine) (Alfa Aesar, Germany), Darvan C (ammonium polymethacrylate) (Vanderbilt Minerals, USA) and Triton X-100 (98–100%) (Merck Millipore).
2.3. Sample collection
The stability and dispersion efficiency of TiO2NPs in two different aqueous matrices were examined in river water and wastewater, spiked with nanoscale anatase and rutile. The Sava River water was collected at Litija (Slovenia) during the first sampling campaign of the EU 7th FW funded GLOBAQUA project32 in September 2014. The wastewater sample was taken from the effluent of Wastewater Treatment Plant Domžale-Kamnik (Slovenia) in November, 2014. Samples were stored in 1 L polyethylene bottles, immediately transported to the laboratory and kept frozen at −20 °C until analysis.2.4. Microwave assisted digestion of TiO2NPs
Decomposition of TiO2NP samples was performed following the two-step microwave assisted digestion procedure, previously developed in our group for the digestion of sediments33 with some modifications. 10.0 ± 0.5 mg of anatase or rutile was weighed into a 100 mL high-pressure Teflon vessel. Into the vessel 4 mL of HNO3, 2 mL of HF and 1 mL of HCl were added. The sample was subjected to closed vessel microwave digestion at maximal power of 1200 W: ramp to temperature 200 °C in 30 min, pressure 50 bar, hold at 200 °C for 60 min, and cooling for 30 min. After that the Teflon vessel was vented and the vessel cap was removed. 6 mL of H3BO3 (4% aqueous solution) was added to dissolve fluorides and to complex the residual HF. In the second step of microwave digestion the following programme was applied: ramp to temperature 200 °C in 15 min, pressure 50 bar, hold at 200 °C for 30 min, and cooling for 30 min. After digestion a clear solution was obtained. The content was quantitatively transferred to a 100 mL graduated glass or perfluoroalkoxy (PFA) Teflon flask and filled to mark with water. The same procedure, with an exception that no TiO2NP sample was added, was applied to determine the blank. The total Ti concentration in the digested sample was determined by ICP-MS. All the experiments were performed in at least three replicates. A similar procedure for decomposing TiO2NPs in cosmetic and food products was also used by Cámara et al.223. Results and discussion
3.1. Determination of total Ti concentrations in TiO2NPs by ICP-MS
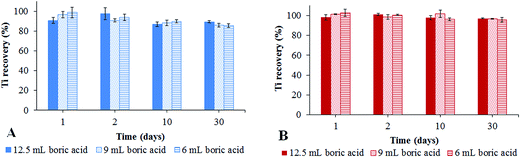
The determination of total Ti concentrations in TiO2NPs is used not only for its quantification, but also for the evaluation of the efficiency of TiO2NP dispersion by the use of different approaches for stabilization of NPs in suspensions. For this purpose, effective sample digestion of refractory TiO2NPs is necessary prior to the determination of the total Ti concentration by ICP-MS. In order to decompose TiO2NPs a rapid digestion procedure by the use of ammonium persulfate as a fusing reagent with reported good recoveries19 was first applied. The Ti concentration in the sample digest was immediately determined by ICP-MS. To avoid possible polyatomic interference from sulphur and nitrogen compounds arising from ammonium persulfate (e.g.32S16O+ and 34S14N+),34 the 49Ti isotope (5.46%) was monitored instead of the 48Ti isotope (73.98%) in the helium collision mode (4.3 mL min−1 He gas flow). The recoveries between the determined and added Ti concentration in TiO2NPs were less than 60% with poor repeatability (50%) of the analytical procedure and were most probably related to inefficient digestion of the samples, non-quantitative transfer of suspensions after the digestion and difficulties in effective cleaning of the beakers in which digestion was performed.To achieve quantitative recoveries with acceptable repeatability, microwave assisted digestion was then applied. Since no certified reference material for TiO2NPs exists, the method for microwave assisted digestion was validated by a spike recovery test using commercially available nanoscale rutile and anatase. The slightly modified procedure previously developed in our group,33 as described in paragraph 2.4., was used to digest anatase and rutile samples. In the optimization of the two step digestion process, the mixture of HNO3, HF and HCl was first added, followed by the addition of various amounts of 4% H3BO3 (6, 9 or 12.5 mL). The addition of H3BO3 is mandatory to complex the residual HF in order to prevent the reaction of HF with the quartz part of the ICP-MS. The digested samples were transferred into 100 mL glass bottles and stored at 4 °C. The total Ti concentration was determined after 1, 2, 10 and 30 days by ICP-MS (monitoring the 48Ti isotope). Prior to Ti determination by ICP-MS, samples were appropriately diluted with water. The results expressed as recoveries between the determined and added Ti concentrations are presented in Fig. 1.
From the data of Fig. 1 it is evident that one day after the digestion the recoveries between determined and added Ti in anatase and rutile in general lie between 97 and 102%, which indicates efficient sample decomposition. The recoveries around 100% were observed for rutile in samples measured 30 days after the digestion, while for anatase the recoveries for Ti gradually decreased with time and were between 85 and 90% 30 days after the digestion. The slight losses of Ti in digested anatase solution are most likely related to the adsorption of Ti ions on the negatively charged glass surfaces35 despite the low pH (<1) of samples. No decrease in the Ti concentration over time is observed for digested nanoscale rutile. Namely, since rutile contains 5 wt% SiO2 as a surface coating, Si ions are also released into solution during the digestion procedure and are adsorbed on the glass surface, hindering Ti ion adsorption. In order to prevent the possibility of Ti ion adsorption on the glass surface, in the following experiments Teflon laboratory ware was applied. The same experiments as shown in Fig. 1 were repeated for digested samples stored in Teflon. The data revealed that the recoveries for anatase and rutile were around 100%, even 30 days after the digestion, when stored in Teflon. In contrast to the hydrophilic glass surface with the negative charge, Teflon (polytetrafluoroethylene) as a hydrophobic polymer does not adsorb Ti ions.36 Data from the above experiments demonstrated that the amount of 4% H3BO3 added in the second step of the digestion procedure did not have any significant influence on the efficiency of anatase and rutile decomposition. Therefore, to minimize the consumption of acids, 6 mL of 4% H3BO3 was further applied and Teflon laboratory ware was used in the following experiments. The limit of detection (LOD) and quantification (LOQ) for the determination of Ti in nanoscale anatase and rutile by ICP-MS after microwave assisted digestion were calculated on the basis of three times and ten times the standard deviation of the signal of nine blank samples analysed, respectively. A low LOD (0.086 ng Ti mL−1) and LOQ (0.287 ng Ti mL−1) were obtained. The linearity of measurement for Ti in nanoscale anatase and rutile by ICP-MS was obtained over the concentration range from LOQ to 100 ng Ti mL−1.
3.2. Determination of total Ti concentrations in aqueous solutions of TiO2NPs by ICP-MS
In order to demonstrate the applicability of the optimized microwave assisted digestion for decomposition of TiO2NPs in aqueous solutions, 10 mg of anatase or rutile were weighed into a Teflon vessel and 20 mL of water was added. The microwave assisted digestion procedure and sample manipulation were performed as described before. For comparison of analytical data, powdered nanoscale TiO2 without the addition of water was analysed under the same analytical procedure. The results of these experiments are presented in Table 2.| Sample | Recovery (%) | Average (%) | RSD (%) | |
|---|---|---|---|---|
| Anatase | A | 95.2 | 98.0 | 3.2 |
| 101.5 | ||||
| 97.3 | ||||
| B | 98.2 | 99.1 | 1.8 | |
| 98.0 | ||||
| 101.2 | ||||
| Rutile | A | 97.6 | 101.1 | 3.5 |
| 101.3 | ||||
| 104.5 | ||||
| B | 100.7 | 100.2 | 0.4 | |
| 99.9 | ||||
| 99.9 | ||||
Data from Table 2 demonstrate that the recoveries between the determined and added Ti concentrations lie between 98 and 101% for nanoscale anatase and rutile when powdered samples were digested, or when 20 mL of water was added to powdered samples before the digestion. This extends the applicability of the analytical procedure for the quantification of nanoscale Ti in environmental waters. From the data of Table 2 it is further evident that good repeatability of results between three replicates with relative standard deviation (RSD ± 3.5%) was obtained.
3.3. Stabilisation of TiO2NPs in aqueous solutions
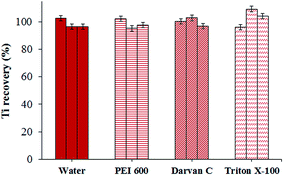
In sizing and quantification of nanoscale Ti22–24,27 it is of crucial importance to maintain efficient dispersion of NPs in solution throughout the analytical procedure. Aggregates of NPs can be separated by introducing the mechanical force or by overcoming weak attractive forces by electrostatic, steric or electrosteric interaction with addition of dispersing agents that adsorb on the surface of particles, suppressing their aggregation.37 In the present work, among the pretreatment methods available for dispersing NPs, different mechanical and ultrasonication procedures in combination with various dispersing agents were tested. As dispersing agents 0.5 wt% cationic polymer PEI 600 (polyethylenimine), 1 wt% anionic dispersant Darvan C (ammonium polymethacrylate) and 1 wt% non-ionic surfactant Triton X-100 (with the hydrophilic polyethyleneoxide chain and hydrophobic aromatic hydrocarbonlipophilic group) were used. First, mechanical shaking (horizontal shaker) in combination with vortex and/or ultrasonic agitation (ultrasonic bath) was applied to aqueous suspension of nanoscale anatase and rutile at different time intervals from 5 to 60 min (mechanical shaking), 0.5 to 1 min (vortex) and 5 to 30 min (ultrasonic bath). The same sample treatments were used for TiO2NP samples dispersed in PEI 600, Darvan C and Triton X-100. After treatment, suspensions were appropriately diluted and 10 mL aliquots were transferred into Teflon vessels. The microwave assisted digestion procedure was applied and concentrations of Ti in the digested sample determined by ICP-MS. The recoveries from 70 to 100% with poor repeatability (RSD ± 30%) were obtained. Lower recoveries are most probably related to the ineffective mechanical shaking of NPs. For improving the dispersing efficiency, an ultrasonic homogenizer with a probe (referred as ultrasonication) was then used for the preparation of suspensions of TiO2NPs in water or in different dispersing agents.In order to test if ultrasonication more efficiently disperses TiO2NPs in aqueous solution than mechanical shaking, vortex or ultrasonic bath, 50 mL dispersions of rutile were prepared by applying 3 min of ultrasonication in water and the use of same dispersing agents. After ultrasonication 10 mL sample aliquots were immediately transferred into Teflon vessels and microwave assisted digestion was applied. The Ti concentrations in digested samples were determined by ICP-MS. The recoveries between the determined and expected Ti concentration are presented in Fig. 2.
As can be seen from Fig. 2, the use of ultrasonication or ultrasonication with different dispersing agents efficiently disperses nanoscale rutile in suspensions, enabling their quantitative transfer into Teflon vessels. Good recoveries ranging from 95 to 105% were obtained. Standard deviations between three replicates for nanoscale rutile dispersed in water, PEI 600, Darvan C and Triton X-100 were ±3.6%, ±3.5%, ±3.0% and ±6.6%, respectively. Slightly worse repeatability using Triton X-100 for dispersion is presumably related to high viscosity of this dispersing agent and to the weaker adsorption of non-ionic Triton X-100 on the charged surfaces of TiO2NPs in comparison to the cationic PEI 600 and anionic Darvan C. The latter two surfactants are more strongly bound to the NP surface due to the presence of ionic groups, resulting in more efficient stabilization of NPs.
To additionally verify the stability of TiO2NP dispersions, zeta potentials were measured in a wide pH range. pH is an important parameter for controlling the NP stability, since it affects the surface charge and particle interactions. Aggregation and sedimentation of NPs in general increases as the pH approaches the point of zero charge – isoelectric point (IEP). The region of low suspension stability typically appears at zeta potential values from −30 to +30 mV, whereas at zeta potentials higher than +30 or lower than −30 mV NPs remain dispersed in suspensions.38
As the most efficient approach for dispersing TiO2NPs in solution, ultrasonication in combination with different dispersing agents was applied in the following experiments. The adsorption of surfactants on the NP surface causes changes in its properties, which results in changing their zeta potential.39 The zeta potentials of nanoscale rutile dispersed by ultrasonication in water, PEI 600, Darvan C or Triton X-100 are presented as a function of pH in Fig. 3.
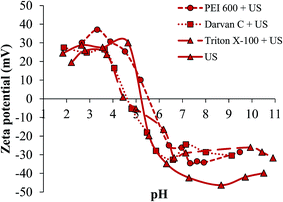 | ||
| Fig. 3 Zeta potentials of nanoscale rutile in MilliQ water dispersed by ultrasonication (US) and the use of different dispersing agents as a function of pH. | ||
From Fig. 3 it can be seen that rutile dispersed in water has IEP at pH 5.3. As expected, PEI 600 slightly shifted IEP toward higher pH (5.6), while Darvan C and Triton X-100 shifted IEP toward lower pH (4.5). NPs are generally stabilized by the adsorption of a dispersant (polyelectrolyte) layer around the particle surface. The positive charge allows PEI to complex with negatively charged TiO2NPs, shifting IEP towards higher pH.40 On the other hand, ammonium salt of polymethacrylic acid (Darvan C) changes the surface charge of TiO2NPs to a more negative value, increasing the electrostatic repulsion between the particles and shifting IEP towards lower pH.41 The same observation can be seen for rutile NPs dispersed in Triton X-100, where hydrophobic chains are adsorbed onto the NP surfaces, while the hydrophilic ethylene oxide chains repulse each other, causing the stabilization of NPs in suspension.42
The results presented in Fig. 3 indicate that the stability of nanoscale rutile dispersed in water, PEI 600, Darvan C or Triton X-100 is similar in acidic pHs, but is higher in alkaline pHs for NPs dispersed only in water. Lower zeta potentials of TiO2NPs dispersed in surfactants are most probably related to the formation of surfactant aggregates on the nanoparticle surface due to the hydrophobic interactions between nonpolar chains of the surfactants. In our experiments, the concentrations of surfactants were significantly lower than their critical micelle concentrations, which leads to the formation of surfactant aggregates.39,43 These aggregates destabilize nanoparticles in suspension and decrease the zeta potential. At the pH of nanoscale rutile suspensions (pH 6.5) the zeta potentials of NPs dispersed in water, PEI 600, Darvan C or Triton X-100 were −38.0, −25.0, −32.7 and −32.4 mV, respectively. It can be concluded that dispersing agents applied do not improve the stability of TiO2NPs in aqueous suspensions. Efficient dispersion of NPs in MilliQ water was already achieved by the use of ultrasonication.
3.4. Stability of TiO2NPs in environmental water samples
The sample matrix of environmental waters (e.g. concentration of NOM, ionic strength) may have significant influence on the stability of TiO2NPs and on the determination of Ti concentrations by ICP-MS. Since concentrations of TiO2NPs in environmental waters were low, spiking of river and wastewater samples was performed. The relevant characteristics of river and wastewater studied are presented in Table 3.| Parameter/sample | pH | Conductivity (μS cm−1) | TOC (mg L−1) | Ca2+ (mg L−1) |
|---|---|---|---|---|
| River water | 7.8 | 279 ± 2 | 3.0 ± 0.2 | 38 ± 1 |
| Wastewater | 7.9 | 854 ± 5 | 30 ± 2 | 68 ± 1 |
As evident, samples investigated have pH close to 8. Since NOM may enhance the stability of NPs, total organic carbon (TOC), which in general originates from decomposition of NOM, was measured in river and wastewater samples. The TOC was 3.0 ± 0.2 mg L−1 in river water and ten times higher in wastewater, while electrical conductivity which is related to the ionic strength, was found to be 279 μS cm−1 and 854 μS cm−1 for river and wastewater, respectively. Ca is one of the major cations, which was present in river (38 mg L−1) and wastewater (68 mg L−1) and importantly contributed to the ionic strength of samples investigated.44 The high Ca content may provoke NP aggregation, and causes severe isobaric interference on the determination of Ti at m/z 48. To avoid this type of interference, Ti concentrations in digested samples were measured at m/z 49, while the He mode was applied to compensate for possible polyatomic interference.
In order to evaluate the influence of the sample matrix on dispersion efficiency of TiO2NPs, 50 mL of river and wastewater samples were spiked with 10 mg of nanoscale rutile or anatase, appropriately diluted and 10 mL aliquots transferred into Teflon vessels. During each dilution step, ultrasonication of the suspensions was applied. Ti concentrations originally present in river and wastewater, and in sample aliquots spiked with TiO2NPs, were determined by ICP-MS after microwave assisted digestion. The recoveries between the determined and expected Ti concentrations are presented in Table 4.
| Sample | Ti concentration (ng mL−1) | Crystalline phase of TiO2NPs added | Ti concentration in TiO2NPs added (ng mL−1) | Ti concentration expected (ng mL−1) | Ti concentration determined (ng mL−1) | Recovery (%) |
|---|---|---|---|---|---|---|
| River water | 74 ± 4 | Rutile | 161 ± 3 | 235 ± 4 | 160 ± 9 | 68 |
| Anatase | 162 ± 1 | 236 ± 4 | 174 ± 12 | 74 | ||
| Wastewater | 2.7 ± 0.1 | Rutile | 155 ± 13 | 158 ± 10 | 81 ± 5 | 51 |
| Anatase | 178 ± 4 | 181 ± 5 | 118 ± 7 | 65 |
As can be seen, the recoveries for anatase and rutile dispersed in river and wastewater samples by the use of ultrasonication are significantly lower (ranging from 51 to 74%) in comparison to quantitative recoveries for rutile dispersed in MilliQ water (Fig. 2, first three columns). Poor recoveries are related to aggregation of TiO2NPs in samples investigated, which prevents quantitative transfer of NP suspensions. Due to the higher ionic strength of wastewater, the extent of TiO2NP aggregation was higher than in river water. The stability of NPs in environmental samples is influenced also by the presence of NOM25 that increases electrostatic and steric repulsions and thus stabilizing NPs.38 Despite higher concentrations of NOM in wastewater, the impact of high ionic strength on NP stability prevailed, which resulted in lower recoveries of digested wastewater samples. Since ultrasonication is not efficient enough to break up aggregates, surfactants need to be used to keep particles stable in suspension.
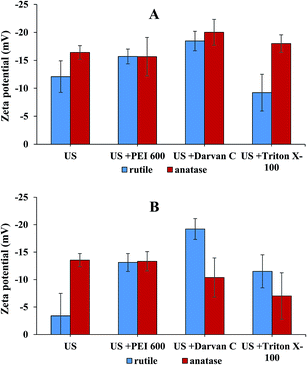
To verify whether the stability of nanoscale anatase and rutile in river and wastewater samples can be improved by the use of dispersing agents, the zeta potentials of TiO2NPs in spiked water samples and spiked water samples with the addition of dispersing agents were measured and are presented in Fig. 4.
From the results in Fig. 4 it can be seen that the zeta potential of nanoscale rutile and anatase dispersed in river and wastewater samples did not exceed the absolute value of 20 mV, indicating that NPs are unstable in dispersions. This is related to the presence of high concentrations of cations that are adsorbed on the particle surface, neutralizing their charge, which leads to smaller electrostatic repulsions between NPs. This effect is more pronounced in wastewater due to the higher ionic strength and for rutile due to the higher specific surface area (according to the manufacturer data, the specific surface area of rutile and anatase is 130–190 m2 g−1 and 45–55 m2 g−1, respectively).13 Rutile NPs with higher surface areas bind cations more effectively. This decreases the stability of NPs and reduces the zeta potentials. The higher degree of aggregation for rutile dispersed in river and wastewater in comparison to anatase is in accordance with lower Ti recoveries of digested rutile samples (Table 4).
From Fig. 4 it is further evident that the use of dispersing agents in combination with ultrasonication does not significantly improve the dispersion efficiency in river or wastewater samples (low zeta potentials), demonstrating the strong influence of matrix constituents on the stability of TiO2NPs in environmental waters.
4. Conclusions
Microwave assisted digestion was optimized for decomposition of powdered nanoscale anatase and rutile and their dispersions in aqueous solutions. By applying the two-step digestion procedure using the mixture of HNO3, HCl and HF in the first, and the addition of H3BO3 in the second step, recoveries close to 100% and good repeatability (RSD ± 3.5%) were obtained. Nanoscale rutile was effectively stabilised in MilliQ water, using ultrasonication for dispersion. In combination with different dispersing agents (PEI 600, Darvan C and Triton X-100), ultrasonication did not significantly improve the stabilization of TiO2NPs in MilliQ water. The efficiency of dispersion was verified by the determination of total Ti concentrations by ICP-MS after the microwave assisted digestion of samples. By applying the same dispersing procedures to river and wastewaters, spiked with nanoscale rutile and anatase, it has been demonstrated that due to the high ionic strength, the stability of NPs was significantly decreased. In wastewater, despite the high content of NOM, the effect of high ionic strength prevailed. TiO2NP aggregation was reflected through low zeta potentials and poor Ti recoveries in the digested water samples.The data of the present investigation contribute to reliable procedures for the digestion and quantitative determination of TiO2NPs in aqueous samples. The study offers effective procedures for stabilization of NPs in aqueous samples that are of significant importance when analytical methods for sizing of nanoscale Ti are applied. In order not to compromise NP original properties, ultrasonication without the use of dispersing agents is recommended for the dispersion of TiO2NPs.
Acknowledgements
This work has been supported by the European Communities 7th Framework Programme Funding under Grant agreement no. 603629-ENV-2013-6.2.1-Globaqua and by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia (Programme group P1-0143).References
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Goetz, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- A. Menard, D. Drobne and A. Jemec, Environ. Pollut., 2011, 159, 677–684 CrossRef CAS PubMed.
- K. Hund-Rinke and M. Simon, Environ. Sci. Pollut. Res., 2006, 13, 225–232 CrossRef CAS PubMed.
- B. Campos, C. Rivetti, P. Rosenkranz, J. M. Navas and C. Barata, Aquat. Toxicol., 2013, 130–131, 174–183 CrossRef CAS PubMed.
- X. Zhu, Y. Chang and Y. Chen, Chemosphere, 2010, 78, 209–215 CrossRef CAS PubMed.
- Z. Clemente, V. L. Castro, C. M. Jonsson and L. F. Fraceto, J. Nanopart. Res., 2014, 16, 2559 CrossRef.
- R. J. Miller, S. Bennett, A. A. Keller, S. Pease and H. S. Lenihan, PLoS One, 2012, 7, 1–7 Search PubMed.
- G. Federici, B. J. Shaw and R. D. Handy, Aquat. Toxicol., 2007, 84, 415–430 CrossRef CAS PubMed.
- W. Fan, M. Cui, H. Liu, C. Wang, Z. Shi, C. Tan and X. Yang, Environ. Pollut., 2011, 159, 729–734 CrossRef CAS PubMed.
- D. B. Warheit, T. R. Webb, K. L. Reed, S. Frerichs and C. M. Sayes, Toxicology, 2007, 230, 90–104 CrossRef CAS PubMed.
- C. Jin, Y. Tang, F. G. Yang, X. L. Li, S. Xu, X. Y. Fan, Y. Y. Huang and Y. J. Yang, Biol. Trace Elem. Res., 2011, 141, 3–15 CrossRef CAS PubMed.
- K. Gerloff, I. Fenoglio, E. Carella, J. Kolling, C. Albrecht, A. W. Boots, I. Förster and R. P. F. Schins, Chem. Res. Toxicol., 2012, 25, 646–655 CrossRef CAS PubMed.
- I. Chowdhury, S. L. Walker and S. E. Mylon, Environ. Sci.: Processes Impacts, 2013, 15, 275–282 CAS.
- G. E. Schaumann, A. Philippe, M. Bundschuh, G. Metreveli, S. Klitzke, D. Rakcheev, A. Grün, S. K. Kumahor, M. Kühn, T. Baumann, F. Lang, W. Manz, R. Schulz and H.-J. Vogel, Sci. Total Environ., 2015, 535, 3–19 CrossRef CAS PubMed.
- B. J. Shaw, C. S. Ramsden, A. Turner and R. D. Handy, Chemosphere, 2013, 92, 1136–1144 CrossRef CAS PubMed.
- H. Sun, X. Zhang, Q. Niu, Y. Chen and J. C. Crittenden, Water, Air, Soil Pollut., 2007, 178, 245–254 CrossRef CAS.
- M. G. A. Korn, A. C. Ferreira, A. C. S. Costa, J. A. Nobrega and C. R. Silva, Microchem. J., 2002, 71, 41–48 CrossRef CAS.
- C. Yong, Adv. Mater. Res., 2013, 718–720, 1113–1117 CrossRef.
- K. Khosravi, M. E. Hoque, B. Dimock, H. Hintelmann and C. D. Metcalfe, Anal. Chim. Acta, 2012, 713, 86–91 CrossRef CAS PubMed.
- J. C. Méranger and E. Somers, Analyst, 1968, 93, 799–801 RSC.
- Z. Sulcek and P. Povondra, Methods of Decomposition in Inorganic Analysis, CRC Press, Boca Raton, FL, 1989 Search PubMed.
- I. López-Heras, Y. Madrid and C. Cámara, Talanta, 2014, 124, 71–78 CrossRef PubMed.
- L. Prieto-Rodriguez, S. Miralles-Cuevas, I. Oller, A. Agüera, G. Li Puma and S. Malato, J. Hazard. Mater., 2012, 211–212, 131–137 CrossRef CAS PubMed.
- C. Contado and A. Pagnoni, Anal. Chem., 2008, 80, 7594–7608 CrossRef CAS PubMed.
- C. Tso, C. Zhung, Y. Shih, Y.-M. Tseng, S. Wu and R. Doong, Water Sci. Technol., 2010, 61, 127–133 CrossRef CAS PubMed.
- D. Rakcheev, A. Philippe and G. E. Schaumann, Anal. Chem., 2013, 85, 10643–10647 CrossRef CAS PubMed.
- F. Laborda, E. Bolea and J. Jimenez-Lamana, Anal. Chem., 2014, 86, 2270–2278 CrossRef CAS PubMed.
- S. M. Majedi, B. C. Kelly and H. K. Lee, Anal. Chim. Acta, 2013, 789, 47–57 CrossRef CAS PubMed.
- F. Loosli, P. Le Coustumer and S. Stoll, Sci. Total Environ., 2015, 535, 28–34 CrossRef CAS PubMed.
- C. Sentein, B. Guizard, S. Giraud, C. Yé and F. Ténégal, J. Phys.: Conf. Ser., 2009, 170, 1–7 Search PubMed.
- P. Leroy, C. Tournassat and M. Bizi, J. Colloid Interface Sci., 2011, 356, 442–453 CrossRef CAS PubMed.
- A. Navarro-Ortega, V. Acuña, A. Bellin, P. Burek and G. Cassiani, et al. , Sci. Total Environ., 2015, 503–504, 3–9 CrossRef CAS PubMed.
- J. Ščančar, T. Zuliani, T. Turk and R. Milačič, Environ. Monit. Assess., 2007, 127, 271–282 CrossRef PubMed.
- T. W. May and R. H. Wiedmeyer, At. Spectrosc., 1998, 19, 150–155 CAS.
- S. H. Behrens and D. G. Grier, J. Chem. Phys., 2001, 115, 6716–6721 CrossRef CAS.
- T. Preočanin, A. Selmani and P. Lindqvist-Reis, et al. , Colloids Surf., A, 2012, 412, 120–128 CrossRef.
- M. H. Nia, M. R. Tavirani and A. R. Nikoofar, et al. , Journal of Paramedical Sciences, 2015, 6, 96–105 Search PubMed.
- B. J. R. Thio, D. Zhou and A. A. Keller, J. Hazard. Mater., 2011, 189, 556–563 CrossRef CAS PubMed.
- N. H. Tkachenko, Z. M. Yaremko, C. Bellmann and M. M. Soltys, J. Colloid Interface Sci., 2006, 299, 686–695 CrossRef CAS PubMed.
- C. Dempsey, I. Lee, K. R. Cowan and J. Suh, Colloids Surf., B, 2013, 112, 108–112 CrossRef CAS PubMed.
- B. P. Singh, S. Nayak, S. Samal, S. Bhattacharjee and L. Besra, Appl. Surf. Sci., 2012, 258, 3524–3531 CrossRef CAS.
- J. Dong, S. Chen, D. S. Corti, E. I. Franses, Y. Zhao, H. T. Ng and E. Hansonet, J. Colloid Interface Sci., 2011, 362, 33–41 CrossRef CAS PubMed.
- Z. M. Yaremko, N. H. Tkachenko, C. Bellman and A. Pich, J. Colloid Interface Sci., 2006, 296, 565–571 CrossRef CAS PubMed.
- A. A. Keller, H. Wang and D. Zhou, et al. , Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |