Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular biosensors based on electropolymerised pyrrole–cyclodextrin modified surfaces for antibody detection
Ewelina
Wajs
,
Núria
Fernández
and
Alex
Fragoso
*
Nanobiotechnology & Bioanalysis Group, Department d'Enginyeria Química, Universitat Rovira i Virgili, Avinguda Països Catalans 26, 43007 Tarragona, Spain. E-mail: alex.fragoso@urv.cat
First published on 6th April 2016
Abstract
The self-assembly of an adamantane-appended polymer bearing an antigen fragment on a polypyrrole–cyclodextrin modified surface provides a highly sensitive immunosensor with low limits of detection for celiac disease related targets. The pyrrole-carboxylic acid films were formed on the surface of gold electrodes by electropolymerisation and followed by covalent attachment of cyclodextrin units. Surface plasmon resonance measurements confirmed the role of the host/guest interactions between adamantane moieties and β-cyclodextrin hosts in the formation of the supramolecular sensor interface. Furthermore, this novel electrochemical supramolecular platform was effective in the amperometric detection of anti-gliadin antibodies in spiked serum samples with very good signal recovery.
Introduction
The construction of nanostructures based on supramolecular interactions is becoming an attractive strategy for the modification of surfaces. The combination of weak interactions such as host–guest, hydrophobic, charge transfer or hydrogen bonding allows the construction of complex architectures with defined and organized structures and sometimes unusual properties.1,2Cyclodextrins (CD) are a family of cyclic supramolecular receptors featuring a central hydrophobic cavity that allows the enclosure of several types of guest molecules of appropriate size to form the so-called “inclusion complex”.3 The cavity of the CD molecule is lined up with a great number of hydroxyl groups, which give the molecule a highly hydrophilic character in its external part. These important properties have encouraged the use of CDs in a range of biotechnological applications such as enzyme technology4 and biosensing5–9 although this field is still very young and promising. The most common method employed for the modification of surfaces with cyclodextrins involves the chemisorption of thiolated cyclodextrin derivatives onto gold surfaces.5,10,11
Cyclodextrins also have been combined with conductive polymers to develop sensors for small molecules. Among the many existing conductive polymers,12 polypyrrole (PPy) has drawn considerable attention due to its good stability under ambient conditions, ease of derivatisation, good redox properties and the ability to give high electrical conductivities, compared with other conjugated materials.13 PPy is easily generated both chemically (by oxidation with Fe3+) and electrochemically. Electropolymerisation of pyrrole monomers leads to the simple and reproducible formation of organic films with precise spatial resolutions and provides an easy control over the polymer thickness.14,15 Derivatised PPy bearing carboxylic acid groups has been used as supports for the covalent attachment of capture antibodies in immunosensors and studied by electrochemical and surface plasmon techniques.16–19
Neutral and anionic cyclodextrins have been incorporated in polypyrrole films. Pyrrole has been electropolymerised in the presence of native β-CD and the resulting functionalized polymer film featured interesting electrochemical properties such as selective, simultaneous and quantitative detection of some organic compounds of interest such as polyhydroxyphenols and neurotransmitters derived from pyrogallol and catechol.20 Similarly, poly(pyrrole-sulfated cyclodextrin) films have been prepared electrochemically from heptasulfonated β-CD and pyrrole21,22 and used for the electro-controlled delivery of neutral drugs.23 The central idea beyond this strategy is to combine the unique electrical, electrochemical and optical properties of these conjugated polymers with the selectivity provided by the supramolecular hosts. This method thus affords thin films in which the CD host provides the surface with a molecular recognition host able to interact with several molecules but has the drawback of a frequent lack of stability and reproducibility in the incorporated layer due to the relatively weak forces that stabilise the composite materials.
This method can be improved by the covalent attachment of the CD host to an electropolymerisable monomer or to an already formed PPy film. It can be anticipated that this linkage generates a more stable monolayer with tunable recognition properties that could be employed in the immobilization of enzymes or antibodies for biosensors, an area in which little progress has been made in spite of the obvious potential they have. This strategy also has the advantage of high compatibility and low non-specific adsorption of matrix proteins due to the hydrophilic character of the surface and the possibility of recycling the CD surface to anchor other architectures by self-assembly due to the reversibility of the host–guest interactions.
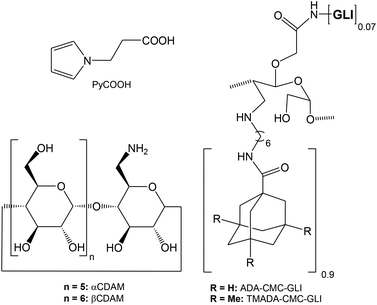
The main goal of this study is the preparation and characterization of polypyrrole–cyclodextrin modified surfaces and their possible application in biosensing, using a celiac disease related antibody as a model analyte24 (Scheme 1). Polypyrrole–cyclodextrin modified electrodes were used as supports for the immobilization of a bifunctionalised polysaccharide carrying adamantane units (for docking to the cyclodextrin surface) and gliadin units (Chart 1). Disposable screen-printed electrodes (SPE) were used as substrates due to their low cost, ease of use and versatility25 and the electrochemical properties and reproducibility of the films were investigated by cyclic voltammetry, ac-impedance spectroscopy and surface plasmon resonance. The application and analytical parameters of the polypyrrole–cyclodextrin modified electrodes in the amperometric detection of autoantibodies related to celiac disease is also described.
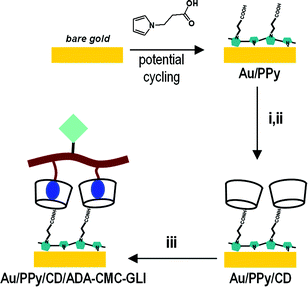 | ||
| Scheme 1 Preparation of biosensor surface. (i) EDC/NHS; (ii) mono-6-amino-6-deoxy- β-CD; (iii) ADA-CMC-GLI. | ||
Experimental
Materials
Carboxymethylcellulose, sodium salt (90 kDa, CMC), 1H-pyrrole-1-propanoic acid (PyCOOH), adamantane (ADA) carboxylic acid, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), 3,3′,5,5′-tetramethylbenzidine (TMB), gliadin, rabbit anti-gliadin polyclonal-IgG-antibody, and anti-rabbit IgG (whole molecule)–peroxidase antibody produced in goat (Ab-HRP) were purchased from Aldrich. β-CD was a gift from Roquette (France). Mono-6-amino-6-deoxy-β-cyclodextrin (CDAM)26 and ADA-CMC-GLI27 were prepared as reported previously.Electrochemical instrumentation
Electrochemical measurements were performed on a PC controlled PGSTAT12 Autolab potentiostat (EcoChemie, The Netherlands) with a built-in frequency response analyser FRA2 module. The electrochemical impedance measurements were performed in 1 mM K3[Fe(CN)6] in 0.15 M KCl at a bias potential of 0.2 V in the frequency range 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–0.1 Hz.
000–0.1 Hz.
Screen-printed electrodes (SPEs) were purchased from DropSens SL (Oviedo, Spain). The SPEs were pre-treated before use with three potential cycles in the range 0–1.6 V at 50 mV s−1 scan rate in sulfuric acid solution (1 M).
Modification of SPEs and amperometric measurements
PyCOOH (10 mM in acetonitrile containing 0.1 M LiClO4) was electropolymerised on the SPEs by applying 10 potential cycles in the range −0.2 V to +1.5 V at 20 mV s−1 to form a PPy film. The electrodes were then washed with acetonitrile and water and used in the next step. The PPy-modified SPEs were immersed into a stirred aqueous solution containing EDC and NHS (5 mg each) and the mixture was stirred for 1 hour at 4 °C. An excess of CDAM (10 mg) was then added and left under stirring overnight. The remaining carboxylic acid groups were neutralised with 1 M ethanolamine for 1 hour. After rinsing with water and drying, 50 μL of a 5 mg mL−1 of ADA-CMC-GLI solution was spotted over the SPEs and left to incubate overnight to form the supramolecular complex.The next incubation steps were carried out immediately prior to the amperometric measurements. For this, 50 μL of target anti-gliadin antibody (0–0.5 μg mL−1) was incubated for 30 minutes over the SPEs. After rinsing with water, 50 μL of Ab-HRP conjugate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) was further incubated for 10 minutes. The amperometric measurements were carried out by first recording the background response at +0.2 V in 10 mM PBS + 0.15 M NaCl (pH 6), followed by the addition of 20 μL of TMB substrate.
1000) was further incubated for 10 minutes. The amperometric measurements were carried out by first recording the background response at +0.2 V in 10 mM PBS + 0.15 M NaCl (pH 6), followed by the addition of 20 μL of TMB substrate.
Surface plasmon resonance (SPR) study of surface modification
SPR measurements were carried out in an Autolab Esprit SPR instrument on gold-coated glass sensors (25 mm) at room temperature in static mode (λ = 670 nm, angle resolution 0.05 m°). The instrument incorporates a combined electrochemistry/SPR cuvette that was connected to a PGSTAT12 Autolab potentiostat in which the gold sensor behaves as both a working electrode and a SPR sensor. After recording the background SPR signal in 10 mM PBS + 0.15 M NaCl (pH 6), the surface of the sensor was electropolymerised with PyCOOH as described before using a Ag/AgCl reference and Pt counter electrodes integrated in the electrochemical/SPR cuvette. After thoroughly washing the cell with acetonitrile and water, the Au/PPy surface was modified in situ with EDC/NHS, CDAM and ethanolamine as described above by flowing the corresponding solutions above the sensor for 1 hour, 6 hours and 30 minutes, respectively, at 20 μL min−1 using an external pump system. After washing again with water the interaction of the Au/PPy/CD surface with ADA/CMC/GLI, CMC/GLI and TMADA/CMC/GLI (5 mg mL−1) was studied by measuring the SPR signal after overnight incubation at room temperature using 10 mM PBS + 0.15 M NaCl (pH 6) as the running buffer.Results and discussion
Electrochemical characterisation of the supramolecular assembly
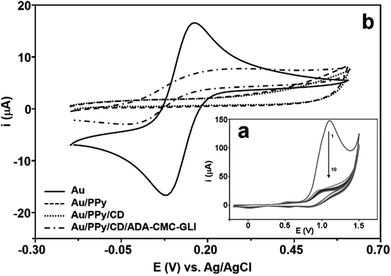
The anodic electropolymerization of PPyCOOH was carried by cyclic voltammetry in the presence of 10 mM of the monomer in LiClO4.28Fig. 1a shows a typical cyclic voltammetric response of the electropolymerisation with increasing potential cycles. Initially, there is a first strong oxidation peak occurring at around +1 V, which is greatly reduced in the following cycles until a steady state is reached. The formation of the PPy film over the gold surface was confirmed by cyclic voltammetry.
Fig. 1b shows the cyclic voltammograms of 1 mM Fe(CN)63− obtained before and after the electropolymerization. The response for the bare gold was typical of a diffusion-controlled process and the absence of a redox signal in the current–potential curve for the Au/PPy electrode indicates that the surface was totally covered by the polymer film, which hinders the electron transfer from the ferricyanide probe resulting in a featureless voltammogram. After modification with the polypyrrole film, the cyclodextrin hosts were attached on the electrodes by a reaction with aminated cyclodextrin to form an amido linkage that was confirmed by the presence of an O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N stretching band at 1678 cm−1 in the FTIR-ATR spectrum. The cyclic voltammogram obtained for the Fe(CN)63− probe after this step was very similar to the response obtained for PPy. After overnight incubation of the Au/PPy/CD electrode in a solution of ADA-CMC-GLI a quasireversible signal is clearly visible at 0.12 V with a peak-to-peak separation of 284 mV (Fig. 1b). This increase in the CV response with respect to the PPy/CD film is due to the cationic character of ADA-CMC-GLI arising from the presence of the aminohexane groups in the CMC backbone.
C–N stretching band at 1678 cm−1 in the FTIR-ATR spectrum. The cyclic voltammogram obtained for the Fe(CN)63− probe after this step was very similar to the response obtained for PPy. After overnight incubation of the Au/PPy/CD electrode in a solution of ADA-CMC-GLI a quasireversible signal is clearly visible at 0.12 V with a peak-to-peak separation of 284 mV (Fig. 1b). This increase in the CV response with respect to the PPy/CD film is due to the cationic character of ADA-CMC-GLI arising from the presence of the aminohexane groups in the CMC backbone.
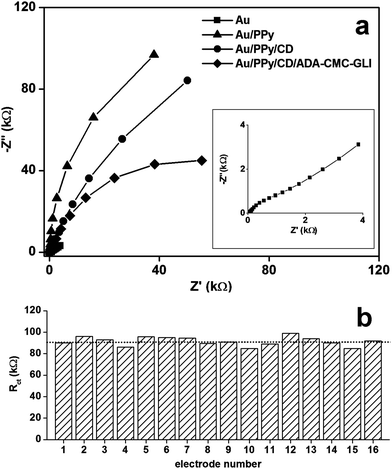
Electrode modification was also studied by impedance spectroscopy (Fig. 2a). The resistance to charge transfer (Rct) obtained from simulation increased from 800 Ω (bare) to 292 kΩ (PPy), indicating the formation of a film on the electrode and is in agreement with the cyclic voltammetric data. The shape of the Nyquist plot for the electropolymerisation with PPy is very similar to other polypyrrole films, which show high slopes indicating high conductivities and a capacitive behavior.29,30
Upon modification with CDAM the Rct value decreases to 179 kΩ while the capacitance of the double layer (Cdl), which measures the tendency of the film to accumulate electrical charge, remained essentially unchanged (14.0 μF for Au/PPy and 14.4 μF for Au/PPy/CD). This suggests that the cyclodextrin molecules are not absorbed within the PPy matrix but are rather located at the surface of the polymer and therefore do not alter the electrical properties of the film. The decrease in Rct is due to the partial replacement of the negative carboxylate groups on the surface by neutral amido groups and also to a higher hydrophilicity given by the cyclodextrin units that allows a better diffusion of the Fe(CN)63− probe toward the electrode. Modification with ADA-CMC-GLI is accompanied by further decrease of Rct up to 90 kΩ.
For practical applications, the preparation of reproducible surfaces is essential, in particular in this case where several deposition steps and polydisperse materials are employed. A study on the reproducibility of the electropolymerization and ADA-CMC-GLI deposition was carried out by impedance spectroscopy. For this, 16 individual working electrodes were subsequently modified to form a complete Au/PPy/CD/ADA-CMC-GLI layer and the Rct value was measured for each of the electrodes. As can be seen, the electropolymerization and ADA-CMC-GLI deposition was highly reproducible with an average Rct of 92 ± 4 kΩ, representing a standard deviation of 4.3% (Fig. 2b).
Surface plasmon resonance study of the formation of the supramolecular surface
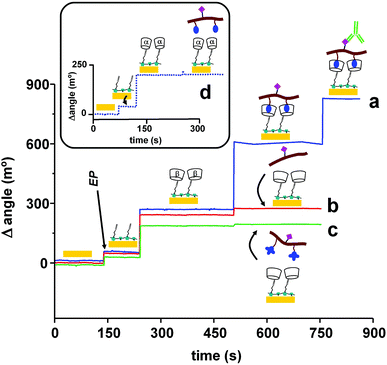
Further evidence of the formation of the multilayered supramolecular structure was obtained from surface plasmon resonance. This technique has been widely used to monitor biomolecular interactions and material depositions over gold surfaces. In our experiments, the SPR instrument was coupled to a potentiostat allowing us to carry out in situ the electropolymerization of PyCOOH without removing the gold chip from the SPR cell. The amount of immobilised molecules after each modification step was evaluated using the formula 1 ng mm−2 is equivalent to 120 millidegrees (m°), as indicated in the instrument user's manual.Fig. 3 shows the plots of the changes in reflected light intensity as a function of the incident angle for the different modification steps. After recording the SPR signal of the bare electrode, the initial modification leading to the formation of the PPy film displaced the intensity minimum by 45 m°, which corresponds to the deposition of 2.7 pmol mm−2 of PyCOOH units. The PPy layer was then activated and treated with CDAM, causing a further increase in the minimum intensity to 200 m°, corresponding to ∼1.5 pmol mm−2 of immobilized CD hosts. This value is in the same order of magnitude as the surface concentration obtained with thiolated cyclodextrins on gold (3.3 pmol mm−2)11 and represents ∼56% substitution of carboxylic groups by amide bonds. The increasing trend in the SPR signal was maintained after the incubations of ADA-CMC-GLI (Δangle = 320 m°, corresponding to ∼0.11 pmol mm−2 of immobilized gliadin residues) and the target anti-gliadin antibody (Δangle = 240 m°, ∼0.012 pmol mm−2), confirming the successful formation of the self-assembled structure.
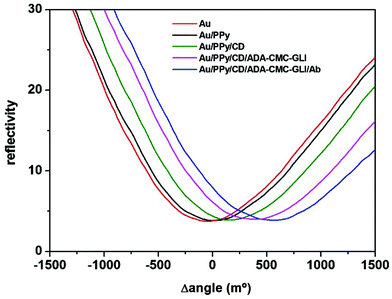 | ||
| Fig. 3 Variations in reflected light intensity (SPR signal) as a function the incidence angle for the different modification steps. | ||
In another experiment, the role of the adamantane units in the formation of the interfacial inclusion complex with the immobilized cyclodextrin hosts was studied (Fig. 4). The first two modification steps (electropolymerisation and cyclodextrin deposition) were identically carried out in all sensors. The similarity of these responses indicates great reproducibility in the covalent attachment of the CD hosts. Then, one sensor was incubated with ADA-CMC-GLI (Fig. 4a), another sensor was incubated with CMC-GLI polymer (i.e. not carrying adamantane units (Fig. 4b)) while the third sensor was incubated with a polymer carrying bulkier tri-methyl-adamantane moieties (Fig. 4c). As can be seen, the incubations of CMC-GLI and TMADA-CMC-GLI caused a very low variation in the SPR signal (33 and 27 m°, respectively) with respect to the ADA-containing polymer. In addition, ADA-CMC-GLI was not immobilised on the surface bearing smaller α-CD hosts (Fig. 4d). These experiments confirm the role of the host/guest interactions between ADA moieties and β-CD hosts in the formation of the supramolecular sensor interface.
Antibody detection on PPy–CD electrodes
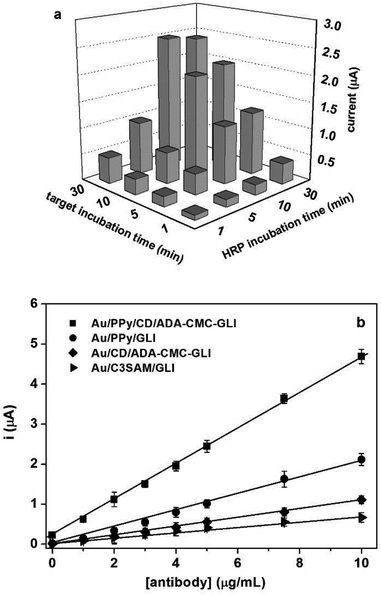
The functionality of the developed self-assembled surface was tested in the development of an amperometric biosensor for the determination of an anti-gliadin antibody based on the formation of a sandwich immunocomplex between the gliadin units immobilised on the ADA-CMC-GLI polymer, the target antibody and a reporter anti-mouse-HRP conjugate. In a previous work by our group,27 we studied the role of the CD/ADA interactions by comparing the analytical performance of four surfaces having different support layers/antigen architectures. The results indicated that a multivalent presentation of both the support layer (containing CDs) and antigen contained in a doubly-modified polymeric scaffold bearing the ADA units to anchor to the surface and resulted in the highest performance of the sensing platform.Fig. 5a shows the influence of the target and reporter incubation times on the amperometric response of the biosensor for 5 μg mL−1 of anti-gliadin antibody. As can be seen, the optimum combination of incubation times was 30 minutes for the target and 10 min for the reporter conjugate. Using these optimum incubation times, a calibration curve was obtained (Fig. 5b). The dependence of the amperometric signal with anti-gliadin antibody concentration was linear in the range 0–10 μg mL−1 with a sensitivity (taken as the slope of the linear fit) of 0.45 μA mL μg−1 and a limit of detection of 33 ng mL−1 (Fig. 5) which is in the same order of magnitude of the biosensor reported by our group for anti-gliadin antibodies.24
To understand the possible influence of both the PPy film and the CD/ADA interactions in the analytical performance, biosensors with three alternative ways of immobilizing the gliadin antigen were prepared and their interfacial concentration was estimated by SPR (Table 1). In one case, the gliadin units were directly attached (via EDC/NHS chemistry) to the Au/PPy surface (Au/PPy/GLI). Another sensor as modified with a self-assembled monolayer (SAM) of hepta-6-thio-6-deoxy-βCD11 onto which the ADA-CMC-GLI polymer was self-assembled (Au/CD/ADA-CMC-GLI). Finally, a control surface was prepared by immobilizing gliadin on a SAM of 3-mercaptopropionic acid thus lacking both PPy and CD moieties (Au/C3SAM/GLI). All these modifications gave similar gliadin surface coverage to Au/PPy/CD/ADA-CMC-GLI. Interestingly, when these surfaces were applied to the amperometric detection of the anti-gliadin antibody as described above, the obtained sensitivities and LOD values were significantly lower (see Table 1), indicating that the combination of the PPy film with the CD/ADA interface plays a crucial role in the sensitivity of the biosensor. This may be a consequence, on the one hand, of the high number of COOH groups on the surface that allows for the immobilisation of a high number of CD anchoring units and, on the other hand, to the porous and permeable structure of PPy films.17,18
The possible effect of the serum matrix on target detection using the Au/PPy/CD/ADA-CMC-GLI biosensor was studied by spiking foetal calf serum with different amounts of anti-gliadin antibody (Table 2). As can be seen, the supramolecular biosensor is able to detect the target antibody in a complex matrix like serum with signal recovery near 100%, suggesting the applicability of the system to the analysis of serum samples.
| Added (μg mL−1) | Found (μg mL−1) | Recovery (%) |
|---|---|---|
| 0.50 | 0.53 | 106 |
| 1.0 | 1.1 | 110 |
| 2.0 | 2.2 | 105 |
| 5.0 | 5.2 | 104 |
Conclusions
The attachment of cyclodextrin hosts on electropolymerised pyrrole-carboxylic acid films represents a novel surface modification approach for the construction of high-performance biosensors. The polypyrrole–cyclodextrin modified electrodes were used as supports for the immobilization of a bifunctionalised polysaccharide carrying adamantane units (for docking to the cyclodextrin surface) and gliadin units on disposable screen-printed electrodes. The films were characterised by electrochemical methods and surface plasmon resonance and applied to the detection of antibodies related to celiac disease with very good sensitivity and low LOD. The developed biosensor was also applied in spiked serum samples with very good signal recovery.Acknowledgements
We thank Ministerio de Ciencia e Innovación, Spain, for financial support (grant BIO2008-02841).References
- L. C. Palmer, Y. S. Velichko, M. O. Cruz and S. I. Stupp, Philos. Trans. R. Soc., A, 2007, 365, 1417 CrossRef CAS PubMed.
- J. Sun, M. Cai and J. J. Lavigne, in Supramolecular Chemistry: From Molecules to Nanomaterials, ed. J. W. Steed and P. A. Gale, John Wiley & Sons, Hoboken, New Jersey, 2012, vol. 4, p. 1825 Search PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743 CrossRef CAS.
- R. Villalonga, R. Cao and A. Fragoso, Chem. Rev., 2007, 107, 3088 CrossRef CAS.
- A. Fragoso, B. Sanromà, M. Ortiz and C. K. O'Sullivan, Soft Matter, 2009, 5, 400 RSC.
- A. Fragoso, J. Caballero, E. Almirall, R. Villalonga and R. Cao, Langmuir, 2002, 18, 5051 CrossRef CAS.
- X. Zhang, L. Wu, J. Zhou, X. Zhang and J. Chen, J. Electroanal. Chem., 2015, 742, 97 CrossRef CAS.
- X. Shi, G. Chen, L. Yuan, Z. Tang, W. Liu, Q. Zhang, D. M. Haddleton and H. Chen, Mater. Horiz., 2014, 1, 540 RSC.
- L. Fritea, M. Tertiş, S. Cosnier, C. Cristea and R. Săndulescu, Int. J. Electrochem. Sci., 2015, 10, 7292 CAS.
- M. J. W. Ludden, M. Péter, D. N. Reinhoudt and J. Huskens, Chem. Soc. Rev., 2006, 35, 1122 RSC.
- M. T. Rojas, R. Koniger, J. F. Stoddart and A. E. Kaifer, J. Am. Chem. Soc., 1995, 117, 336 CrossRef CAS.
- T. K. Das and S. Prusty, Polym.-Plast. Technol. Eng., 2012, 51, 1487 CrossRef CAS.
- L.-X. Wang, X.-G. Li and Y.-L. Yang, React. Funct. Polym., 2001, 47, 125 CrossRef CAS.
- J. Bobacka, A. Ivaska and A. Lewenstam, Electroanalysis, 2003, 15, 366 CrossRef CAS.
- S. Sadki, P. Schottland, N. Brodie and G. Sabouraud, Chem. Soc. Rev., 2000, 29, 283 RSC.
- V. Serafín, L. Agüí, P. Yáñez-Sedeño and J. M. Pingarrón, Biosens. Bioelectron., 2014, 52, 98 CrossRef PubMed.
- H. Dong, Ch. M. Li, W. Chen, Q. Zhou, Z. X. Zeng and J. H. T. Luong, Anal. Chem., 2006, 78, 7424 CrossRef CAS PubMed.
- H. Dong, X. Cao, Ch. M. Li and W. Hu, Biosens. Bioelectron., 2008, 23, 1055 CrossRef CAS.
- W. Hu, Ch. M. Li, X. Cui, H. Dong and Q. Zhou, Langmuir, 2007, 23, 2761 CrossRef CAS PubMed.
- N. Izaoumen, D. Bouchta, H. Zejli, M. El Kaoutit, A. M. Stalcup and K. R. Temsamani, Talanta, 2005, 66, 111 CrossRef CAS PubMed.
- K. R. Temsamani, H. B. Mark, W. Kutner and A. M. Stalcup, J. Solid State Electrochem., 2002, 6, 391 CrossRef CAS.
- W. Chen, X. Wan, N. Xu and G. Xue, Macromolecules, 2003, 36, 276 CrossRef CAS.
- G. Bidan, C. Lopez, F. Mendes-Viegas, E. Vieil and A. Gadelle, Biosens. Bioelectron., 1995, 10, 219 CrossRef CAS.
- E. Wajs, F. Caldera, F. Trotta and A. Fragoso, Analyst, 2014, 139, 375 RSC.
- H. M. Nassef, L. Civit, A. Fragoso and C. K. O'Sullivan, Analyst, 2008, 133, 1736 RSC.
- A. Fragoso, R. Cao and R. Villalonga, J. Carbohydr. Chem., 1995, 14, 1379 CrossRef CAS.
- M. Ortiz, A. Fragoso and C. K. O'Sullivan, Anal. Chem., 2011, 83, 2931 CrossRef CAS.
- S. M. Sayyah, S. S. El-Rehim and M. M. El-Deeb, J. Appl. Polym. Sci., 2003, 90, 1783 CrossRef CAS.
- K. H. An, K. K. Heo, S. C. Lim, D. J. Bae and Y. H. Lee, J. Electrochem. Soc., 2002, 149, 1058 CrossRef.
- R. B. Moghaddama and P. G. Pickup, Phys. Chem. Chem. Phys., 2010, 12, 4733 RSC.
| This journal is © The Royal Society of Chemistry 2016 |