Rapid and improved characterization of therapeutic antibodies and antibody related products using IdeS digestion and subunit analysis
Abstract
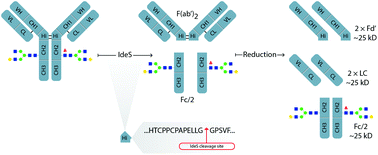
The immunoglobulin degrading enzyme from Streptococcus pyogenes, IdeS, was discovered as a mechanism by which pathogenic bacteria circumvent antibody mediated immune defense. IdeS was found to rapidly and specifically cleave IgG into F(ab′)2 and Fc/2 fragments. The enzymatic specificity has led to a range of recent developments in the analytical strategies for characterization of monoclonal therapeutic antibodies and related products such as antibody-drug conjugates, bispecific antibodies, antibody mixtures and Fc-fusion proteins. In this review article we describe the discovery and properties of IdeS, discuss the current challenges in characterizing antibody therapeutics and review the methodologies using IdeS to improve the characterization of therapeutic antibodies. The review is focused on critical quality attributes of the final antibody product as studied by IdeS fragmentation such as Fab- and Fc-glycosylation, oxidation, glycation, C-terminal lysine and others. In summary, this review presents a wide range of IdeS-based applications for improved characterization of originator, biosimilar and next generation antibody-based therapeutics.

 Please wait while we load your content...
Please wait while we load your content...