Highly selective radical isothiocyano-chalcogenization of alkenes with NH4SCN in water†
Abstract
In the presence of catalytic amounts of molecular iodine and stoichiometric potassium persulfate, a green, highly chemoselective, regioselective and cis-selective radical isothiocyano-chalcogenization of alkenes with NH4SCN in water is disclosed. This three component reaction features high selectivities, an environmentally benign process, mild conditions, high yields, excellent functional-group tolerance, and broad substrate scope. The resulting products can be further transformed into other molecules with structural similarities of these compounds to bioactive analogs.
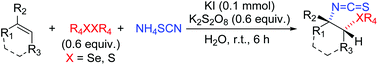


 Please wait while we load your content...
Please wait while we load your content...